Lumbar Fusion for Spinal Instability and Degenerative Disc Conditions, Including Sacroiliac Fusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evicore Spine Imaging Guidelines
CLINICAL GUIDELINES Spine Imaging Policy Version 1.0 Effective February 14, 2020 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2017 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2019 eviCore healthcare. All rights reserved. Spine Imaging Guidelines V1.0 Spine Imaging Guidelines Procedure Codes Associated with Spine Imaging 3 SP-1: General Guidelines 5 SP-2: Imaging Techniques 14 SP-3: Neck (Cervical Spine) Pain Without/With Neurological Features (Including Stenosis) and Trauma 22 SP-4: Upper Back (Thoracic Spine) Pain Without/With Neurological Features (Including Stenosis) and Trauma 26 SP-5: Low Back (Lumbar Spine) Pain/Coccydynia without Neurological Features 28 SP-6: Lower Extremity Pain with Neurological Features (Radiculopathy, Radiculitis, or Plexopathy and Neuropathy) With or Without Low Back (Lumbar Spine) Pain 32 SP-7: Myelopathy 36 SP-8: Lumbar Spine Spondylolysis/Spondylolisthesis 39 SP-9: Lumbar Spinal Stenosis 42 SP-10: Sacro-Iliac (SI) Joint Pain, Inflammatory Spondylitis/Sacroiliitis and Fibromyalgia 44 SP-11: Pathological Spinal Compression Fractures 47 SP-12: Spinal Pain in Cancer Patients 49 SP-13: Spinal Canal/Cord Disorders (e.g. -

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -

Etiopathogenesis of Sacroiliitis
Korean J Pain 2020;33(4):294-304 https://doi.org/10.3344/kjp.2020.33.4.294 pISSN 2005-9159 eISSN 2093-0569 Review Article Etiopathogenesis of sacroiliitis: implications for assessment and management Manuela Baronio1, Hajra Sadia2, Stefano Paolacci3, Domenico Prestamburgo4, Danilo Miotti5, Vittorio A. Guardamagna6, Giuseppe Natalini1, and Matteo Bertelli3,7,8 1Dipartimento di Anestesia, Rianimazione, Terapia Intensiva e del Dolore, Fondazione Poliambulanza, Brescia, Italy 2Atta-ur-Rahman School of Applied Biosciences, National University of Science and Technology, Islamabad, Pakistan 3MAGI’s Lab, Rovereto, Italy 4Ortopedia e Traumatologia, Ospedali Civili di Legnano e Cuggiono, Cuggiono, Italy 5Cure Palliative e Terapia del Dolore, ICS Maugeri, Pavia, Italy 6Cure Palliative e Terapia del Dolore, IRCCS IEO, Milano, Italy 7MAGI Euregio, Bolzano, Italy 8EBTNA-LAB, Rovereto, Italy Received January 16, 2020 Revised March 17, 2020 The sacroiliac joints connect the base of the sacrum to the ilium. When inflamed, Accepted April 16, 2020 they are suspected to cause low back pain. Inflammation of the sacroiliac joints is called sacroiliitis. The severity of the pain varies and depends on the degree of Handling Editor: Kyung Hoon Kim inflammation. Sacroiliitis is a hallmark of seronegative spondyloarthropathies. The presence or absence of chronic sacroiliitis is an important clue in the diagnosis of Correspondence low back pain. This article aims to provide a concise overview of the anatomy, physi- Stefano Paolacci ology, and molecular biology of sacroiliitis to aid clinicians in the assessment and MAGI’s Lab, Via delle Maioliche, 57/D, management of sacroiliitis. For this narrative review, we evaluated articles in Eng- Rovereto, Trentino 38068, Italy lish published before August 2019 in PubMed. -

New ASAS Criteria for the Diagnosis of Spondyloarthritis: Diagnosing Sacroiliitis by Magnetic Resonance Imaging 9
Document downloaded from http://www.elsevier.es, day 10/02/2016. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiología. 2014;56(1):7---15 www.elsevier.es/rx UPDATE IN RADIOLOGY New ASAS criteria for the diagnosis of spondyloarthritis: ଝ Diagnosing sacroiliitis by magnetic resonance imaging ∗ M.E. Banegas Illescas , C. López Menéndez, M.L. Rozas Rodríguez, R.M. Fernández Quintero Servicio de Radiodiagnóstico, Hospital General Universitario de Ciudad Real, Ciudad Real, Spain Received 17 January 2013; accepted 10 May 2013 Available online 11 March 2014 KEYWORDS Abstract Radiographic sacroiliitis has been included in the diagnostic criteria for spondy- Sacroiliitis; loarthropathies since the Rome criteria were defined in 1961. However, in the last ten years, Diagnosis; magnetic resonance imaging (MRI) has proven more sensitive in the evaluation of the sacroiliac Magnetic resonance joints in patients with suspected spondyloarthritis and symptoms of sacroiliitis; MRI has proven imaging; its usefulness not only for diagnosis of this disease, but also for the follow-up of the disease and Axial spondy- response to treatment in these patients. In 2009, The Assessment of SpondyloArthritis inter- loarthropathies national Society (ASAS) developed a new set of criteria for classifying and diagnosing patients with spondyloarthritis; one important development with respect to previous classifications is the inclusion of MRI positive for sacroiliitis as a major diagnostic criterion. This article focuses on the radiologic part of the new classification. We describe and illustrate the different alterations that can be seen on MRI in patients with sacroiliitis, pointing out the limitations of the technique and diagnostic pitfalls. -

Brown Tumors, Presenting with a Degenerative Lumber Disc Like Pain
Open Access Archives of Pathology and Clinical Research Case Report A great mimicker of Bone Secondaries: Brown Tumors, presenting with a ISSN 2640-2874 Degenerative Lumber Disc like pain Zuhal Bayramoglu1*, Ravza Yılmaz1 and Aysel Bayram2 1Department of Radiology, Istanbul Medical Faculty, Istanbul University, Capa, Fatih, 34093, Istanbul, Turkey 2Department of Pathology, Istanbul Medical Faculty, Istanbul University, Capa, Fatih, 34093, Istanbul, Turkey *Address for Correspondence: Dr. Zuhal ABSTRACT Bayramoglu, Department of Radiology, Istanbul Medical Faculty, Istanbul University, Capa, Fatih, 34093, Istanbul, Turkey, Tel: +90-212- 414-20-00, This report presents an adult patient suffering from sacroiliitis like low back pain, lumbosacral radiculopathy Fax: +90-212-631-07-28; Email: and elbow swelling. Multimodality imaging revealed multiple lytic bone lesions located in supra acetabular [email protected] iliac bone, sacrum, and distal end of radius. Painful numerous lesions due to the extension to the articular Submitted: 06 June 2017 surfaces are not expected for Brown tumors. Less than ten cases with multiple Brown tumor due to primary Approved: 14 July 2017 hyperparathyroidism has been reported. Although Brown tumors are mostly diagnosed incidentally, this case Published: 17 July 2017 would awake the physicians about rheumatological symptoms in the presentation of Brown tumors. Since Brown tumors are non-touch bone lesions that are expected to regress after parathyroid adenoma removal, it is Copyright: 2017 Bayramoglu Z. This is an important to distinguish Brown tumors from the giant cell tumors. open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in CASE PRESENTATION any medium, provided the original work is properly cited. -
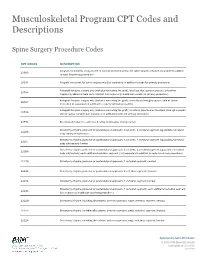
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Clinical Guidelines
CLINICAL GUIDELINES Interventional Pain Management Services Version 1.0.2019 Clinical guidelines for medical necessity review of comprehensive musculoskeletal management services. © 2019 eviCore healthcare. All rights reserved. Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 Interventional Pain Management CMM-200: Epidural Steroid Injections (ESI) 3 CMM-201: Facet Joint Injections/Medial Branch Blocks 17 CMM-202: Trigger Point Injections 21 CMM-203: Sacroiliac Joint Injections 32 CMM-204: Prolotherapy 37 CMM-207: Epidural Adhesiolysis 40 CMM-208: Radiofrequency Joint Ablations/Denervations 44 CMM-209: Regional Sympathetic Blocks 51 CMM 210: Implantable Intrathecal Drug Delivery Systems 57 CMM-211: Spinal Cord Stimulators 65 CMM-308: Thermal Intradiscal Procedures 66 CMM-310: Manipulation of the Spine Under Anesthesia 71 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 2 of 73 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-200: Epidural Steroid Injections (ESI) CMM-200.1: Definitions 4 CMM-200.2: General Guidelines 5 CMM-200.3: Indications: Selective Nerve Root Block (SNRB) 6 CMM-200.4: Indications: Epidural Steroid Injections 7 CMM-200.5: Non-Indications: SNRB 8 CMM-200.6: Non-Indications: ESI 8 ® CMM-200.7: Procedure (CPT ) Codes 9 CMM-200.8: References 10 ______________________________________________________________________________________________________ -

TESSYS Technique with Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED with Intact Articular Process : an In-Silico Study
TESSYS Technique With Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED With Intact Articular Process : An In-Silico Study Jingchi Li West China Hospital/West China School of Medicine for Sichuan University Chen Xu Changzheng Hospital Aliated to the Naval Medical University Xiaoyu Zhang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Zhipeng Xi Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Mengnan Liu Macau University of Science and Technology Zhongxin Fang Xihua University Nan Wang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Lin Xie ( [email protected] ) Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Yueming Song West China Hospital/West China School of Medicine for Sichuan University Research Article Keywords: Biomechanical deterioration, Transforaminal endoscopic discectomy, Endoscopic dynamic drill, Facetectomy, Iatrogenic annulus injury Posted Date: April 26th, 2021 Page 1/27 DOI: https://doi.org/10.21203/rs.3.rs-429749/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at BMC Musculoskeletal Disorders on July 10th, 2021. See the published version at https://doi.org/10.1186/s12891-021-04504-1. Page 2/27 Abstract Background: The facetectomy was reported as an important procedure in both in-out and out-in (i.e. transforaminal endoscopic spine system (TESSYS)) techniques in the transforaminal endoscopic discectomy (TED), and which was also related to the deterioration of postoperative biomechanical environment and related poor prognosis. -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020)
Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020) Procedure Code Description ACL Repair 27407 Repair, primary, torn ligament and/or capsule, knee; cruciate ACL Repair 27409 Repair, primary, torn ligament and/or capsule, knee; collateral and cruciate ligaments ACL Repair 29888 Arthroscopically aided anterior cruciate ligament repair/augmentation or reconstruction Acromioplasty and Rotator Cuff Repair 23130 Acromioplasty Or Acromionectomy, Partial, With Or Without Coracoacromial Ligament Release Acromioplasty and Rotator Cuff Repair 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Acromioplasty and Rotator Cuff Repair 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; chronic Acromioplasty and Rotator Cuff Repair 23415 Coracoacromial Ligament Release, With Or Without Acromioplasty Acromioplasty and Rotator Cuff Repair 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Arthroscopy, Shoulder, Surgical; Decompression Of Subacromial Space With Partial Acromioplasty, With Coracoacromial Ligament (Ie, Arch) Release, When Performed (List Separately In Addition Acromioplasty and Rotator Cuff Repair 29826 To Code For Primary Procedure) Acromioplasty and Rotator Cuff Repair 29827 Arthroscopy, shoulder, surgical; with rotator cuff repair Allograft for Spinal Fusion [BMP] 20930 Allograft, morselized, or placement of osteopromotive material, for spine surgery only Ankle Fusion 27870 Arthrodesis, ankle, open Ankle Fusion -

Acquired Degenerative Changes of the Intervertebral Segments at And
European Journal of Radiology 50 (2004) 134–158 Acquired degenerative changes of the intervertebral segments at and suprajacent to the lumbosacral junction A radioanatomic analysis of the nondiscal structures of the spinal column and perispinal soft tissues J. Randy Jinkins a,b,∗ a Department of Radiologic Sciences, Downstate Medical Center, State University of New York, Brooklyn, NY 11203, USA b Fonar Corporation, 110 Marcus Drive, Melville, NY 11747, USA Received 3 October 2003; received in revised form 9 October 2003; accepted 13 October 2003 Abstract A review of the imaging features of normal and degenerative anatomy of the spine on medical imaging studies shows features that have been largely overlooked or poorly understood by the imaging community in recent years. The imaging methods reviewed included computed tomography (CT) with multiplanar reconstructions and magnetic resonance imaging (MRI). A routine part of the MRI examination included fat-suppressed T2 weighted fast-spin- or turbo-spin-echo acquisitions. As compared to the normal features in asymptomatic volunteers, alterations in the observed CT/MRI morphology and MR signal characteristics were sought in symptomatic individuals. Findings in symptomatic subjects which departed from the normal anatomic features of the posterior spinal elements in asymptomatic volunteers included: rupture of the interspinous ligament(s), neoarthrosis of the interspinous space with perispinous cyst formation, posterior spinal facet (zygapophyseal joint) arthrosis, related central spinal canal, lateral recess (subarticular zone) and neural foramen stenosis, posterior element alterations associated with various forms of spondylolisthesis, and perispinal muscle rupture/degeneration. These findings indicate that the posterior elements are major locations of degenerative spinal and perispinal disease that may accompany or even precede degenerative disc disease. -

Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy
Y Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines 1 G Evidence-Based Clinical Guidelines for Multidisciplinary ETHODOLO Spine Care M NE I DEL I U /G ON Diagnosis and Treatment of I NTRODUCT Lumbar Disc I Herniation with Radiculopathy NASS Evidence-Based Clinical Guidelines Committee D. Scott Kreiner, MD Paul Dougherty, II, DC Committee Chair, Natural History Chair Robert Fernand, MD Gary Ghiselli, MD Steven Hwang, MD Amgad S. Hanna, MD Diagnosis/Imaging Chair Tim Lamer, MD Anthony J. Lisi, DC John Easa, MD Daniel J. Mazanec, MD Medical/Interventional Treatment Chair Richard J. Meagher, MD Robert C. Nucci, MD Daniel K .Resnick, MD Rakesh D. Patel, MD Surgical Treatment Chair Jonathan N. Sembrano, MD Anil K. Sharma, MD Jamie Baisden, MD Jeffrey T. Summers, MD Shay Bess, MD Christopher K. Taleghani, MD Charles H. Cho, MD, MBA William L. Tontz, Jr., MD Michael J. DePalma, MD John F. Toton, MD This clinical guideline should not be construed as including all proper methods of care or excluding or other acceptable methods of care reason- ably directed to obtaining the same results. The ultimate judgment regarding any specific procedure or treatment is to be made by the physi- cian and patient in light of all circumstances presented by the patient and the needs and resources particular to the locality or institution. I NTRODUCT 2 Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines I ON Financial Statement This clinical guideline was developed and funded in its entirety by the North American Spine Society (NASS). All participating /G authors have disclosed potential conflicts of interest consistent with NASS’ disclosure policy.