A Novel Μct Analysis Reveals Different Responses of Bioerosion and Secondary Accretion to Environmental Variability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patterns in Marine Community Assemblages on Continental Margins: a Faunal and Floral Synthesis from Northern Western Australian Atolls
Journal of the Royal Society of Western Australia, 94: 267–284, 2011 Patterns in marine community assemblages on continental margins: a faunal and floral synthesis from northern Western Australian atolls A Sampey 1 & J Fromont 2 1 Aquatic Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, WA 6986 [email protected] 2 Aquatic Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, WA 6986 [email protected] Manuscript received November 2010; accepted January 2011 Abstract Corals and fishes are the most visually apparent fauna on coral reefs and the most often monitored groups to detect change. In comparison, data on noncoral benthic invertebrates and marine plants is sparse. Whether patterns in diversity and distribution for other taxonomic groups align with those detected in corals and fishes is largely unknown. Four shelf-edge atolls in the Kimberley region of Western Australia were surveyed for marine plants, sponges, scleractinian corals, crustaceans, molluscs, echinoderms and fishes in 2006, with a consequent 1521 species reported. Here, we provide the first community level assessment of the biodiversity of these atolls based on these taxonomic groups. Four habitats were surveyed and each was found to have a characteristic community assemblage. Different species assemblages were found among atolls and within each habitat, particularly in the lagoon and reef flat environments. In some habitats we found the common taxa groups (fishes and corals) provide adequate information for community assemblages, but in other cases, for example in the intertidal reef flats, these commonly targeted groups are far less useful in reflecting overall community patterns. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Marine Biodiversity Survey of Mermaid Reef (Rowley Shoals), Scott and Seringapatam Reef Western Australia 2006 Edited by Clay Bryce
ISBN 978-1-920843-50-2 ISSN 0313 122X Scott and Seringapatam Reef. Western Australia Marine Biodiversity Survey of Mermaid Reef (Rowley Shoals), Marine Biodiversity Survey of Mermaid Reef (Rowley Shoals), Scott and Seringapatam Reef Western Australia 2006 2006 Edited by Clay Bryce Edited by Clay Bryce Suppl. No. Records of the Western Australian Museum 77 Supplement No. 77 Records of the Western Australian Museum Supplement No. 77 Marine Biodiversity Survey of Mermaid Reef (Rowley Shoals), Scott and Seringapatam Reef Western Australia 2006 Edited by Clay Bryce Records of the Western Australian Museum The Records of the Western Australian Museum publishes the results of research into all branches of natural sciences and social and cultural history, primarily based on the collections of the Western Australian Museum and on research carried out by its staff members. Collections and research at the Western Australian Museum are centred on Earth and Planetary Sciences, Zoology, Anthropology and History. In particular the following areas are covered: systematics, ecology, biogeography and evolution of living and fossil organisms; mineralogy; meteoritics; anthropology and archaeology; history; maritime archaeology; and conservation. Western Australian Museum Perth Cultural Centre, James Street, Perth, Western Australia, 6000 Mail: Locked Bag 49, Welshpool DC, Western Australia 6986 Telephone: (08) 9212 3700 Facsimile: (08) 9212 3882 Email: [email protected] Minister for Culture and The Arts The Hon. John Day BSc, BDSc, MLA Chair of Trustees Mr Tim Ungar BEc, MAICD, FAIM Acting Executive Director Ms Diana Jones MSc, BSc, Dip.Ed Editors Dr Mark Harvey BSC, PhD Dr Paul Doughty BSc(Hons), PhD Editorial Board Dr Alex Baynes MA, PhD Dr Alex Bevan BSc(Hons), PhD Ms Ann Delroy BA(Hons), MPhil Dr Bill Humphreys BSc(Hons), PhD Dr Moya Smith BA(Hons), Dip.Ed. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -

Hermatypic Coral Fauna of Subtropical Southeast Africa: a Checklist!
Pacific Science (1996), vol. 50, no. 4: 404-414 © 1996 by University of Hawai'i Press. All rights reserved Hermatypic Coral Fauna of Subtropical Southeast Africa: A Checklist! 2 BERNHARD RrnGL ABSTRACT: The South African hermatypic coral fauna consists of 96 species in 42 scleractinian genera, one stoloniferous octocoral genus (Tubipora), and one hermatypic hydrocoral genus (Millepora). There are more species in southern Mozambique, with 151 species in 49 scleractinian genera, one stolo niferous octocoral (Tubipora musica L.), and one hydrocoral (Millepora exaesa [Forskal)). The eastern African coral faunas of Somalia, Kenya, Tanzania, Mozambique, and South Africa are compared and Southeast Africa dis tinguished as a biogeographic subregion, with six endemic species. Patterns of attenuation and species composition are described and compared with those on the eastern boundaries of the Indo-Pacific in the Pacific Ocean. KNOWLEDGE OF CORAL BIODIVERSITY in the Mason 1990) or taxonomically inaccurate Indo-Pacific has increased greatly during (Boshoff 1981) lists of the corals of the high the past decade (Sheppard 1987, Rosen 1988, latitude reefs of Southeast Africa. Sheppard and Sheppard 1991 , Wallace and In this paper, a checklist ofthe hermatypic Pandolfi 1991, 1993, Veron 1993), but gaps coral fauna of subtropical Southeast Africa, in the record remain. In particular, tropical which includes the southernmost corals of and subtropical subsaharan Africa, with a Maputaland and northern Natal Province, is rich and diverse coral fauna (Hamilton and evaluated and compared with a checklist of Brakel 1984, Sheppard 1987, Lemmens 1993, the coral faunas of southern Mozambique Carbone et al. 1994) is inadequately docu (Boshoff 1981). -

Atoll Research Bulletin No* 264 an Annotated Check List of the Corals of American Samoa
ATOLL RESEARCH BULLETIN NO* 264 AN ANNOTATED CHECK LIST OF THE CORALS OF AMERICAN SAMOA BY SEPTEMBER 1983 AN ANNOTATED CHECK LIST OF THE CORALS OF AMERICAN SAMOA by Austin E. Lamberts* SUMMARY Reef coral collections from American Samoa are in the National Museum of Natural History, Smithsonian Institution, Washington, D.C., and in the Hessisches Landesmuseum, Darmstadt, W. Germany. The author has a collection of 790 coral specimens for a total of 1547 items known to be from American Samoa. A total of 177 species (including 3 species of non-scleractinian corals) belonging to 48 genera and subgenera (including the genera Millepora and Heliopora) known to date are listed with data as of frequency of occurrence and habitat, INTRODUCTION The territory of American Samoa comprises the six eastern islands of the Samoan archipelago. It is located in the tropical central south pacific (14's latitude, 170~~longitude) about 2300 nautical miles (4420 km) southwest of Hawaii and 80 miles (130 km) southeast of Western Samoa, Five of the islands are volcanic in origin and are aligned along the crest of a discontinuous submarine ridge which extends over 300 miles (480 km) and tends roughly northwest by southeast. My collecting was done on the five major inhabited islands of American Samoa which the largest, Tutuila, Aunu"u (a small island Located L mi (Le6 km) off the southeast coast of Tutuila), Ofu, Olesega, and Taau. The latter three islands are collectively referred to as the Manu"a group and lie about 46 miles (105 km) east of Tutuila, An uninhabired coral atoll, Rose Island is located LOO mi (161 km) east of Tutuila, One other island, Swains Atoll, is considered part of the Samoan group but is geographically a part of the Tokelau Island group and is not included in this study. -
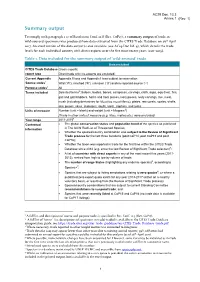
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Scleractinian Corals of the Montebello Islands
Records of the Western Australian Museum Supplement No. 59: 15-19 (2000). SCLERACTINIAN CORALS OF THE MONTEBELLO ISLANDS L.M. Marsh Western Australian Museum, Frands Street, Perth, Western Australia 6000, Australia Summary Montebellos and a further nine from previous The present report lists the first extensive collection records, giving a total of 150 species identified of corals from the Montebello Islands; it is likely that (Table 4). Several species of Montipora, Acropora, many species remain to be found. A total of 141 Favia and Favites remain unidentified and the coral species of 54 genera are recorded from the present fauna is probably still far from completely known, survey and a further 9 species are added from perhaps another 20-30% remains to be discovered. previous records. The coral fauna includes a suite of Seven species of ahermatypic (non zooxanthellate) five genera characteristic of turbid inshore waters but corals were also collected. most of the corals are characteristic of moderately It is probable that many more species will be clear water conditions. The coral fauna overall is found at the Montebellos when the reefs are more most similar to that of the Dampier Archipelago. completely surveyed as many uncommon species may not have been encountered. The species richness of Acropora and Montipora is likely to be Introduction under-recorded because of their great diversity, The first published record of a coral from the polymorphism and taxonomic difficulty. Montebello Islands is by Totton (1952) who figured A suite of species characteristic of upper reef a specimen of Moseleya latistellata collected by Mr fronts exposed to strong wave action (Pocillopora T.H. -

Two New Species of Astreopora (Cnidaria, Anthozoa, Scleractinia) from the Mid-Pacific!
Pacific Science (1980), vol. 34, no. 3 © 1981 by The University Press of Hawaii. All rights reserved Two New Species of Astreopora (Cnidaria, Anthozoa, Scleractinia) from the Mid-Pacific! AUSTIN E. LAMBERTS 2 THE GENUS Astreopora de Blainville 1830, an encrusting reef coral, is found throughout the Indo-Pacific, east to the Tuamotus, and is represented in all major coral collections from the Indo-Pacific (Wells 1954). In shal low water it is often inconspicuous and never predominates. At times it may form heads 2 m or more across, and in deeper waters it may be very common. About eight species can be readily identified and to these two previously unreported species are herein de scribed. Type specimens have been deposited in the Bernice P. Bishop Museum, Honolulu. SPECIES DESCRIPTION Astreopora cucullata species novum Figures 1-4 The original specimen was chiseled from the edge of a 30-cm low, rounded colony embedded on the reef slope in 3 m clear water at the end of the Aua line in Pago Pago harbor, Tutuila, American Samoa, 27 July 1973 (Dahl and Lamberts 1977). Rock substrate with 25 percent coral cover was amid strong currents. Coenosarc .rusty brown in situ. Holotype measures 13+ x 8 x 4cm; weight 142.7 g; volume 132.1 cm 3 . Corallites rather evenly spaced, round or distorted ovals with an average diameter of 1.5 mm. At times two or three openings are FIGURE I. Astreopora eueullata species novum: ho lotype, t natural size. separated by a single thecal wall. Never more than 5 mm between adjacent calix centers and about 6.2 corallites per square Similar studies show the more common centimeter. -

The Bioeroding Sponge Cliona Orientalis Will Not Tolerate Future Projected Ocean Warming Received: 9 February 2018 Blake D
www.nature.com/scientificreports OPEN The bioeroding sponge Cliona orientalis will not tolerate future projected ocean warming Received: 9 February 2018 Blake D. Ramsby 1,2,3, Mia O. Hoogenboom 1, Hillary A. Smith 2,3, Steve Whalan 4 & Accepted: 15 May 2018 Nicole S. Webster 2,3,5 Published: xx xx xxxx Coral reefs face many stressors associated with global climate change, including increasing sea surface temperature and ocean acidifcation. Excavating sponges, such as Cliona spp., are expected to break down reef substrata more quickly as seawater becomes more acidic. However, increased bioerosion requires that Cliona spp. maintain physiological performance and health under continuing ocean warming. In this study, we exposed C. orientalis to temperature increments increasing from 23 to 32 °C. At 32 °C, or 3 °C above the maximum monthly mean (MMM) temperature, sponges bleached and the photosynthetic capacity of Symbiodinium was compromised, consistent with sympatric corals. Cliona orientalis demonstrated little capacity to recover from thermal stress, remaining bleached with reduced Symbiodinium density and energy reserves after one month at reduced temperature. In comparison, C. orientalis was not observed to bleach during the 2017 coral bleaching event on the Great Barrier Reef, when temperatures did not reach the 32 °C threshold. While C. orientalis can withstand current temperature extremes (<3 °C above MMM) under laboratory and natural conditions, this species would not survive ocean temperatures projected for 2100 without acclimatisation or adaptation (≥3 °C above MMM). Hence, as ocean temperatures increase above local thermal thresholds, C. orientalis will have a negligible impact on reef erosion. Increasing global temperatures are requiring organisms to acclimate to greater thermal extremes, migrate, or suf- fer reduced ftness and, potentially, local extirpation. -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of