Tech Memo Marine Biology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patterns in Marine Community Assemblages on Continental Margins: a Faunal and Floral Synthesis from Northern Western Australian Atolls
Journal of the Royal Society of Western Australia, 94: 267–284, 2011 Patterns in marine community assemblages on continental margins: a faunal and floral synthesis from northern Western Australian atolls A Sampey 1 & J Fromont 2 1 Aquatic Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, WA 6986 [email protected] 2 Aquatic Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, WA 6986 [email protected] Manuscript received November 2010; accepted January 2011 Abstract Corals and fishes are the most visually apparent fauna on coral reefs and the most often monitored groups to detect change. In comparison, data on noncoral benthic invertebrates and marine plants is sparse. Whether patterns in diversity and distribution for other taxonomic groups align with those detected in corals and fishes is largely unknown. Four shelf-edge atolls in the Kimberley region of Western Australia were surveyed for marine plants, sponges, scleractinian corals, crustaceans, molluscs, echinoderms and fishes in 2006, with a consequent 1521 species reported. Here, we provide the first community level assessment of the biodiversity of these atolls based on these taxonomic groups. Four habitats were surveyed and each was found to have a characteristic community assemblage. Different species assemblages were found among atolls and within each habitat, particularly in the lagoon and reef flat environments. In some habitats we found the common taxa groups (fishes and corals) provide adequate information for community assemblages, but in other cases, for example in the intertidal reef flats, these commonly targeted groups are far less useful in reflecting overall community patterns. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Scleractinian Reef Corals: Identification Notes
SCLERACTINIAN REEF CORALS: IDENTIFICATION NOTES By JACKIE WOLSTENHOLME James Cook University AUGUST 2004 DOI: 10.13140/RG.2.2.24656.51205 http://dx.doi.org/10.13140/RG.2.2.24656.51205 Scleractinian Reef Corals: Identification Notes by Jackie Wolstenholme is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. TABLE OF CONTENTS TABLE OF CONTENTS ........................................................................................................................................ i INTRODUCTION .................................................................................................................................................. 1 ABBREVIATIONS AND DEFINITIONS ............................................................................................................. 2 FAMILY ACROPORIDAE.................................................................................................................................... 3 Montipora ........................................................................................................................................................... 3 Massive/thick plates/encrusting & tuberculae/papillae ................................................................................... 3 Montipora monasteriata .............................................................................................................................. 3 Massive/thick plates/encrusting & papillae ................................................................................................... -

Pleistocene Reefs of the Egyptian Red Sea: Environmental Change and Community Persistence
Pleistocene reefs of the Egyptian Red Sea: environmental change and community persistence Lorraine R. Casazza School of Science and Engineering, Al Akhawayn University, Ifrane, Morocco ABSTRACT The fossil record of Red Sea fringing reefs provides an opportunity to study the history of coral-reef survival and recovery in the context of extreme environmental change. The Middle Pleistocene, the Late Pleistocene, and modern reefs represent three periods of reef growth separated by glacial low stands during which conditions became difficult for symbiotic reef fauna. Coral diversity and paleoenvironments of eight Middle and Late Pleistocene fossil terraces are described and characterized here. Pleistocene reef zones closely resemble reef zones of the modern Red Sea. All but one species identified from Middle and Late Pleistocene outcrops are also found on modern Red Sea reefs despite the possible extinction of most coral over two-thirds of the Red Sea basin during glacial low stands. Refugia in the Gulf of Aqaba and southern Red Sea may have allowed for the persistence of coral communities across glaciation events. Stability of coral communities across these extreme climate events indicates that even small populations of survivors can repopulate large areas given appropriate water conditions and time. Subjects Biodiversity, Biogeography, Ecology, Marine Biology, Paleontology Keywords Coral reefs, Egypt, Climate change, Fossil reefs, Scleractinia, Cenozoic, Western Indian Ocean Submitted 23 September 2016 INTRODUCTION Accepted 2 June 2017 Coral reefs worldwide are threatened by habitat degradation due to coastal development, 28 June 2017 Published pollution run-off from land, destructive fishing practices, and rising ocean temperature Corresponding author and acidification resulting from anthropogenic climate change (Wilkinson, 2008; Lorraine R. -

Xiv. Stony Corals and Hydrocorals
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository INVERTEBRATE FAUNA OF THE INTERTIDAL ZONE Title OF THE TOKARA ISLANDS -XIV. STONY CORALS AND HYDROCORALS- Author(s) Utinomi, Huzio PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1956), 5(3): 339-346 Issue Date 1956-12-20 URL http://hdl.handle.net/2433/174567 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University INVERTEBRATE FAUNA OF THE INTERTIDAL ZONE OF THE TOKARA ISLANDS XIV. STONY CORALS AND HYDROCORALS1l 2l Huzio UTINOMI Seto Marine Biological Laboratory, Sirahama With Plates XXXI-XXXII The weak development of living reef corals along the raised reefs in the Tokara Islands, where is approximately the northernmost limit of real coral reefs (30°N. Lat.), has been noted by TOKIOKA (1953) and BABA (1954), who gave a brief sketch of the reefs and the inhabitants living there with many excellent photographs. As they noted, living corals usually grow only at crevices and holes which are found here and there on the raised reef covered by purplish Melobesiae, or at the reef margin under low tide level. So there can be seen weakly developed coral reef, particularly at Takarazima. Dr. TOKIOKA, in describing the outline of the shore and its life, recognized the occurrence of only 9 species of the living corals. The present paper presents the results of a re-examination of Dr. TOKIOKA's collection, all deposited in the collections of the Seto Marine Biological Laboratory, and a few additional materials collected by Dr. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -

Hermatypic Coral Fauna of Subtropical Southeast Africa: a Checklist!
Pacific Science (1996), vol. 50, no. 4: 404-414 © 1996 by University of Hawai'i Press. All rights reserved Hermatypic Coral Fauna of Subtropical Southeast Africa: A Checklist! 2 BERNHARD RrnGL ABSTRACT: The South African hermatypic coral fauna consists of 96 species in 42 scleractinian genera, one stoloniferous octocoral genus (Tubipora), and one hermatypic hydrocoral genus (Millepora). There are more species in southern Mozambique, with 151 species in 49 scleractinian genera, one stolo niferous octocoral (Tubipora musica L.), and one hydrocoral (Millepora exaesa [Forskal)). The eastern African coral faunas of Somalia, Kenya, Tanzania, Mozambique, and South Africa are compared and Southeast Africa dis tinguished as a biogeographic subregion, with six endemic species. Patterns of attenuation and species composition are described and compared with those on the eastern boundaries of the Indo-Pacific in the Pacific Ocean. KNOWLEDGE OF CORAL BIODIVERSITY in the Mason 1990) or taxonomically inaccurate Indo-Pacific has increased greatly during (Boshoff 1981) lists of the corals of the high the past decade (Sheppard 1987, Rosen 1988, latitude reefs of Southeast Africa. Sheppard and Sheppard 1991 , Wallace and In this paper, a checklist ofthe hermatypic Pandolfi 1991, 1993, Veron 1993), but gaps coral fauna of subtropical Southeast Africa, in the record remain. In particular, tropical which includes the southernmost corals of and subtropical subsaharan Africa, with a Maputaland and northern Natal Province, is rich and diverse coral fauna (Hamilton and evaluated and compared with a checklist of Brakel 1984, Sheppard 1987, Lemmens 1993, the coral faunas of southern Mozambique Carbone et al. 1994) is inadequately docu (Boshoff 1981). -

FDM 2017 Coral Species Reef Survey
Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific Final ® FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT Dr. Jessica Carilli, SSC Pacific Mr. Stephen H. Smith, SSC Pacific Mr. Donald E. Marx Jr., SSC Pacific Dr. Leslie Bolick, SSC Pacific Dr. Douglas Fenner, NOAA August 2018 Prepared for U.S. Navy Pacific Fleet Commander Pacific Fleet 250 Makalapa Drive Joint Base Pearl Harbor Hickam Hawaii 96860-3134 Space and Naval Warfare Systems Center Pacific Technical Report number 18-1079 Distribution Statement A: Unlimited Distribution 1 Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Washington Headquarters Service, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington, DC 20503. PLEASE DO NOT RETURN YOUR FORM TO THE ABOVE ADDRESS. 1. REPORT DATE (DD-MM-YYYY) 2. REPORT TYPE 3. DATES COVERED (From - To) 08-2018 Monitoring report September 2017 - October 2017 4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT 5b. -

AC26 Doc. 20 Annex 6 English Only / Únicamente En Inglés / Seulement En Anglais
AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais Annex 6 CORAL SPECIES, SYNONYMS, AND NOMENCLATURE REFERENCES CURRENTLY RECOGNIZED IN THE UNEP-WCMC DATABASE Data kindly provided by UNEP-WCMC AC26 Doc. 20, Annex 6 – 1 AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families FamName Accepted Name SpcAuthor Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Acropora mangarevensis; Madrepora ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) crassa; Madrepora abrotanoides ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Acropora diffusa; Madrepora acuminata; ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Madrepora diffusa; Madrepora nigra ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora anthocercis; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) coronata ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Acropora cribripora; Acropora hebes; Acropora manni; Acropora yaeyamaensis; ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Madrepora aspera; Madrepora cribripora; Madrepora hebes; Madrepora manni ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, -

Atoll Research Bulletin No* 264 an Annotated Check List of the Corals of American Samoa
ATOLL RESEARCH BULLETIN NO* 264 AN ANNOTATED CHECK LIST OF THE CORALS OF AMERICAN SAMOA BY SEPTEMBER 1983 AN ANNOTATED CHECK LIST OF THE CORALS OF AMERICAN SAMOA by Austin E. Lamberts* SUMMARY Reef coral collections from American Samoa are in the National Museum of Natural History, Smithsonian Institution, Washington, D.C., and in the Hessisches Landesmuseum, Darmstadt, W. Germany. The author has a collection of 790 coral specimens for a total of 1547 items known to be from American Samoa. A total of 177 species (including 3 species of non-scleractinian corals) belonging to 48 genera and subgenera (including the genera Millepora and Heliopora) known to date are listed with data as of frequency of occurrence and habitat, INTRODUCTION The territory of American Samoa comprises the six eastern islands of the Samoan archipelago. It is located in the tropical central south pacific (14's latitude, 170~~longitude) about 2300 nautical miles (4420 km) southwest of Hawaii and 80 miles (130 km) southeast of Western Samoa, Five of the islands are volcanic in origin and are aligned along the crest of a discontinuous submarine ridge which extends over 300 miles (480 km) and tends roughly northwest by southeast. My collecting was done on the five major inhabited islands of American Samoa which the largest, Tutuila, Aunu"u (a small island Located L mi (Le6 km) off the southeast coast of Tutuila), Ofu, Olesega, and Taau. The latter three islands are collectively referred to as the Manu"a group and lie about 46 miles (105 km) east of Tutuila, An uninhabired coral atoll, Rose Island is located LOO mi (161 km) east of Tutuila, One other island, Swains Atoll, is considered part of the Samoan group but is geographically a part of the Tokelau Island group and is not included in this study. -
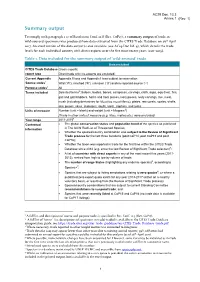
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g.