Thermal Degradation and Biodiesel Production Using Camellia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essential Wholesale & Labs Carrier Oils Chart
Essential Wholesale & Labs Carrier Oils Chart This chart is based off of the virgin, unrefined versions of each carrier where applicable, depending on our website catalog. The information provided may vary depending on the carrier's source and processing and is meant for educational purposes only. Viscosity Absorbtion Comparible Subsitutions Carrier Oil/Butter Color (at room Odor Details/Attributes Rate (Based on Viscosity & Absorbotion Rate) temperature) Description: Stable vegetable butter with a neutral odor. High content of monounsaturated oleic acid and relatively high content of natural antioxidants. Offers good oxidative stability, excellent Almond Butter White to pale yellow Soft Solid Fat Neutral Odor Average cold weather stability, contains occlusive properties, and can act as a moistening agent. Aloe Butter, Illipe Butter Fatty Acid Compositon: Palmitic, Stearic, Oleic, and Linoleic Description: Made from Aloe Vera and Coconut Oil. Can be used as an emollient and contains antioxidant properties. It's high fluidiy gives it good spreadability, and it can quickly hydrate while Aloe Butter White Soft Semi-Solid Fat Neutral Odor Average being both cooling and soothing. Fatty Acid Almond Butter, Illipe Butter Compostion: Linoleic, Oleic, Palmitic, Stearic Description: Made from by combinging Aloe Vera Powder with quality soybean oil to create a Apricot Kernel Oil, Broccoli Seed Oil, Camellia Seed Oil, Evening Aloe Vera Oil Clear, off-white to yellow Free Flowing Liquid Oil Mild musky odor Fast soothing and nourishing carrier oil. Fatty Acid Primrose Oil, Grapeseed Oil, Meadowfoam Seed Oil, Safflower Compostion: Linoleic, Oleic, Palmitic, Stearic Oil, Strawberry Seed Oil Description: This oil is similar in weight to human sebum, making it extremely nouirshing to the skin. -
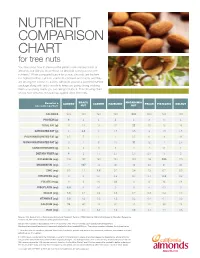
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Is Peanut Butter Keto-Friendly?
Is Peanut Butter Keto-Friendly? Yes! Peanut butter contains carbohydrates, fat, and protein; the ratios of those macronutrients are what makes it acceptable for a keto diet. Our Once Again peanut butters that are made with one simple ingredient: peanuts, are a healthy addition to your keto diet. Peanuts are in the legume family, which includes a variety of beans. Generally speaking, legumes are not included in keto diets; however, peanuts have a higher fat content than other beans which makes them keto-friendly. Moreover, peanuts have a similar macronutrient distribution when compared to nuts such as almonds. They are high in fat and lower in carbs, and thus make a perfect food for the keto lifestyle. Peanut butter fits nicely into your keto diet as a dip for celery, or add it to smoothies, or experiment with it as an ingredient in salad dressings. Is Almond Butter Keto- Friendly? Yes! Almond butter is a staple for most following a keto lifestyle. Used often as a versatile ingredient in salad dressings, dips, and sauces, and in many other culinary innovations, nut butters in general have the desired macronutrient ration for a keto diet, and almond butter is a particularly good choice because of its fiber and vitamin E content. Most of our almond butters contain one simple ingredient: almonds. The newest addition to our product line is our Blanched Almond Butter, available in both in Extra Creamy and Crunchy varieties. Once Again Blanched Almond Butters contain only one gram of carbohydrate per serving! Blanching almonds involves removing the exterior of the almond, leaving behind a surprisingly nutty-sweet interior, with its lighter texture and color. -

Tea Seed Oil and Health Properties Fatih Seyis1, Emine
Tea Seed Oil and Health Properties Fatih Seyis1, Emine Yurteri1, Aysel Özcan1 1Recep Tayyip Erdoğan University: Faculty of Agriculture and Natural Science, Field Crops Department, Rize/Turkey, e-mail: [email protected] Abstract: Tea Oil has a mild fragrant flavor that goes with anything. It’s not a heavy oil like Olive Oil, but thinner – more like almond oil. If the taste or “oiliness” of olive oil overpowers your food. Along with its mild taste and pleasant tea-like aroma, this oil touts impressive health benefits. Tea seed oil has a high smoke point, contains more monounsaturated fatty acids than olive oil, contains fewer saturated fatty acids than olive oil, contains high levels of Vitamin E, polyphenol antioxidants and both Omegas 3 and 6, but has less Omega 6 and Polyunsaturated Fats than olive oil. Health Benefits of tea seed oil are: it can be applied topically and consumed internally to obtain its health benefits, camellia oil can be used for skin, hair, has anti-cancer effects, effects boost immunity and reduces oxidative stress. Camellia oil is used for a variety of other purposes, for example for cooking, as machinery lubricant, as ingredient in beauty products like night creams, salves, in hair care products and perfumes and is used to coat iron products to prevent rusting. Key words: Tea, seed oil, health 1. Introduction Like other genera of Camellia (from Theaceae family), the tea plant (C.sinensis) produces large oily seeds. In some countries where tea seed oil is abundantly available, it has been accepted as edible oil (Sahari et al., 2004). -

Juniperus Communis L.) Essential Oil
Antioxidants 2014, 3, 81-98; doi:10.3390/antiox3010081 OPEN ACCESS antioxidants ISSN 2076-3921 www.mdpi.com/journal/antioxidants Article Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism Martina Höferl 1,*, Ivanka Stoilova 2, Erich Schmidt 1, Jürgen Wanner 3, Leopold Jirovetz 1, Dora Trifonova 2, Lutsian Krastev 4 and Albert Krastanov 2 1 Department of Pharmaceutical Chemistry, Division of Clinical Pharmacy and Diagnostics, University of Vienna, Vienna 1090, Austria; E-Mails: [email protected] (E.S.); [email protected] (L.J.) 2 Department Biotechnology, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mails: [email protected] (I.S.); [email protected] (D.T.); [email protected] (A.K.) 3 Kurt Kitzing Co., Wallerstein 86757, Germany; E-Mail: [email protected] 4 University Laboratory for Food Analyses, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +43-1-4277-55555; Fax: +43-1-4277-855555. Received: 11 December 2013; in revised form: 26 January 2014 / Accepted: 28 January 2014 / Published: 24 February 2014 Abstract: The essential oil of juniper berries (Juniperus communis L., Cupressaceae) is traditionally used for medicinal and flavoring purposes. As elucidated by gas chromatography/flame ionization detector (GC/FID) and gas chromatography/mass spectrometry (GC/MS methods), the juniper berry oil from Bulgaria is largely comprised of monoterpene hydrocarbons such as α-pinene (51.4%), myrcene (8.3%), sabinene (5.8%), limonene (5.1%) and β-pinene (5.0%). -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -

Olive Oil Jars Left Behind By
live oil jars left behind by the ancient Greeks are testament to our centuries- old use of cooking oil. Along with salt and pepper, oil Oremains one of the most important and versatile tools in your kitchen. It keeps food from sticking to pans, adds flavor and moisture, and conducts the heat that turns a humble stick of potato into a glorious french fry. Like butter and other fats, cooking oil also acts as a powerful solvent, unleashing fat-soluble nutrients and flavor compounds in everything from tomatoes and onions to spices and herbs. It’s why so many strike recipes begin with heating garlic in oil rather than, say, simmering it in water. The ancient Greeks didn’t tap many cooking oils. (Let’s see: olive oil, olive oil, or—ooh, this is exciting!—how about olive oil?) But you certainly can. From canola to safflower to grapeseed to walnut, each oil has its own unique flavor (or lack thereof), aroma, and optimal cooking temperature. Choosing the right kind for the task at hand can save you money, boost your health, and improve your cooking. OK, so you probably don’t stop to consider your cooking oil very often. But there’s a surprising amount to learn about What’s this? this liquid gold. BY VIRGINIAWILLIS Pumpkin seed oil suspended in corn oil—it looks like a homemade Lava Lamp! 84 allrecipes.com PHOTOS BY KATE SEARS WHERE TO store CANOLA OIL GRAPESEED OIL are more likely to exhibit the characteristic YOUR OIL flavor and aroma of their base nut or seed. -

<I>Carthamus Tinctorius</I>
Promoting the conservation and use of underutilized and neglected crops. 7. SafflowerSafflower Carthamus tinctorius L. Li Dajue and Hans-Henning Mündel netic t Ge Res lan ou P rc al e n s o I ti n a s t n i r t u e t t e n I IPGRI 2 Safflower. Carthamus tinctorius L. The International Plant Genetic Resources Institute (IPGRI) is an autonomous inter- national scientific organization operating under the aegis of the Consultative Group on International Agricultural Research (CGIAR). The international status of IPGRI is conferred under an Establishment Agreement which, by December 1995, had been signed by the Governments of Australia, Belgium, Benin, Bolivia, Burkina Faso, Cameroon, China, Chile, Congo, Costa Rica, Côte d’Ivoire, Cyprus, Czech Republic, Denmark, Ecuador, Egypt, Greece, Guinea, Hungary, India, Iran, Israel, Italy, Jordan, Kenya, Mauritania, Morocco, Pakistan, Panama, Peru, Poland, Portugal, Romania, Russia, Senegal, Slovak Republic, Sudan, Switzerland, Syria, Tunisia, Turkey, Ukraine and Uganda. IPGRI’s mandate is to advance the conservation and use of plant genetic resources for the benefit of present and future generations. IPGRI works in partner- ship with other organizations, undertaking research, training and the provision of scientific and technical advice and information, and has a particularly strong programme link with the Food and Agriculture Organization of the United Nations. Financial support for the agreed research agenda of IPGRI is provided by the Gov- ernments of Australia, Austria, Belgium, Canada, China, Denmark, France, Ger- many, India, Italy, Japan, the Republic of Korea, Mexico, the Netherlands, Norway, Spain, Sweden, Switzerland, the UK and the USA, and by the Asian Development Bank, IDRC, UNDP and the World Bank. -

Honey Recipes on Across the Fence
Across the Fence Honey Recipes – February 2008 Tips for Cooking with Honey Keep honey in a closed container in a dry place. Do not refrigerate, as refrigeration hastens the formation of crystals. Should this granulation occur, place the container in a pan of hot water until all crystals melt. The honey container should not rest on the bottom of the pan. Granulation does not alter the color or flavor of honey. To substitute honey for sugar, use the same amount of honey, but reduce other liquids by ¼ cup. When measuring honey, coat the measuring cup or spoon with oil or melted butter, or measure shortening before measuring honey. If recipe gets too brown, cover with foil to prevent burning. Baked goods made with honey stay fresh longer since honey tends to prevent them from drying out. This is an important factor when baking ahead or when sending baked items to out-of-town family or friends. Lyn Jarvis’ Recipes Apricot Honey Chicken 4 boneless skinless chicken breast halves 3 Tbsp. apricot preserves (5 oz. each) 2 Tbsp. orange juice 1 Tbsp. canola oil 4 tsp. honey In a large skillet, cook chicken in oil over medium heat for 7 to 9 minutes on each side or until juices run clear. Combine the preserves, orange juice, and honey; pour over chicken. Cook for 2 minutes or until heated through. Yield: 4 servings. Peach and Pear Crisp 1 medium fresh peach, peeled and chopped 1½ tsp. lemon juice 1 medium ripe pear, peeled and chopped ¼ tsp. ground cinnamon 1 Tbsp. honey ⅛ tsp. -

Rabbi Eliyahu Shuman Director of Supervision Effective Through 11
June 2, 2021 22 Sivan 5781 La Tourangelle, Inc. 2340 East Main Street Suite 400 Woodland, CA 95776 This is to certify that the 39 products specified in the listing below, manufactured by LA TOURANGELLE, INC. of the above address, are Kosher and under our supervision. PLEASE NOTE THE FOLLOWING CONDITIONS OF CERTIFICATION: All products listed below are Pareve. All products listed below are certified only when bearing the Star-K symbol. All products listed below are Kosher for year-round use, excluding Passover. This letter of certification is valid through November 30, 2021 and is subject to renewal at that time. BRAND: La Tourangelle UKD# PRODUCT LISTING SK0HZG4N550 All Purpose Baking Spray SKDZZE3SG6W Avocado Oil SKVLKXREYAU Avocado Oil Non-Stick Cooking Spray SKB0NQF2TZU AvoCoco Oil SK5KPHXJ7WR Everyday Avocado Oil SK27270H2EA Expeller Pressed Vegetable Oil SK3YJF27B06 Grape Seed Oil SKZR5EBNPJE Grapeseed Oil Non-Stick Cooking Spray SKTA059CQ2D Organic Canola Oil SKEVY33L343 Organic Canola Oil Non-Stick Cooking Spray SKK08UW6IKZ Organic Expeller Pressed & Refined Coconut Oil SKAC2BUNNSR Organic Extra Virgin Coconut Oil SKN6ZPNS4QU Organic Extra Virgin Olive Oil SKIVAMH6Y1J Organic Extra Virgin Olive Oil Non-Stick Cooking Spray SKYKMZA08PT Organic Flaxseed Oil SKSDDTU4VXP Organic Refined Coconut Oil SK8C58TF7N1 Organic Sun Coco Oil SKGNEZ4A2KZ Organic Sunflower Oil SKR3YHL7L04 Organic Toasted Sunflower Oil SKWVAF83EW0 Organic Virgin Unrefined Coconut Oil SKQ4DM4KBR7 Pan Asian Stir Fry Oil Rabbi Eliyahu Shuman Effective Through 11/30/2021 Director of Supervision Page 1 of 2 June 2, 2021 22 Sivan 5781 La Tourangelle, Inc. 2340 East Main Street Suite 400 Woodland, CA 95776 PLEASE NOTE THE FOLLOWING CONDITIONS OF CERTIFICATION: All products listed below are Pareve. -

Chemical Composition of Cold Pressed Brazilian Grape Seed
a ISSN 0101-2061 (Print) ISSN 1678-457X (Online) Food Science and Technology DDOI http://dx.doi.org/10.1590/1678-457X.08317 Chemical composition of cold pressed Brazilian grape seed oil Fernanda Branco SHINAGAWA1, Fernanda Carvalho de SANTANA1, Elias ARAUJO1, Eduardo PURGATTO1, Jorge MANCINI-FILHO1* Abstract Grape seed oil (GSO) is an important by-product of the wine-making industry which has received attention as an alternative source of vegetable oils; its chemical compounds can be influenced by agricultural practices and industrial processing. Knowledge of the composition of Brazilian GSO is scarce; thus, this study aimed to analyze the chemical characteristics, as well as the antioxidant activity of these oils. GSO samples were obtained from Brazilian markets and showed significantly high amounts of phenolic, γ-tocotrienol and phytosterols as well as, the presence of several volatile compounds. Based on these results, is possible to show that oils exhibited good antioxidant activity. Therefore, it can be inferred that Brazilian GSO had a considerable content of phytochemical compounds with biological activity, which allows its association with other vegetable oils. Keywords: seed oils; micronutrients; antioxidant activity. Practical Application: In this study Brazilian grape seed oils were found to have potential to be used for some industrial sectors, such as food ingredients and cosmetics industry. They showed high amount of polyunsaturated fatty acid and significant amount of vitamin E, phenolics, phytosterols and volatile compounds. The knowledge regarding the composition of the products is important once they are made from a sustainable way. 1 Introduction Recent data from the Food and Agriculture Organization order to expand the knowledge of its characteristics and infer of the United Nations (2014) shows that Brazil is the eleventh its potential for human health. -

Fats Ebook Feb 02.Pdf
2 DRHYMAN.COM Contents Contents INTRODUCTION ................................. 8 PART I ........................................... 11 Dietary Fats: The Good, Bad and the Ugly ............................................ 11 Fatty Acids ............................................................................................ 11 Saturated Fat ........................................................................................ 12 Polyunsaturated Fats ............................................................................ 14 Essential Fatty Acids 101- Omega-3 and Omega-6 ............................... 14 The Beneficial Omega-6 Fatty Acid: GLA ............................................... 16 How Fatty Acids Affect Brain Health ..................................................... 17 Omega-7 Fatty Acids ............................................................................ 18 Monounsaturated Fat ............................................................................ 18 Trans Fats ............................................................................................. 20 Trans Fats and Health ........................................................................... 21 Toxins in Fat .......................................................................................... 22 A Case for Organic ................................................................................ 23 DRHYMAN.COM 3 PART II .......................................... 24 Animal Fats .......................................................................