Chemical Composition of Cold Pressed Brazilian Grape Seed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fats Ebook Feb 02.Pdf
2 DRHYMAN.COM Contents Contents INTRODUCTION ................................. 8 PART I ........................................... 11 Dietary Fats: The Good, Bad and the Ugly ............................................ 11 Fatty Acids ............................................................................................ 11 Saturated Fat ........................................................................................ 12 Polyunsaturated Fats ............................................................................ 14 Essential Fatty Acids 101- Omega-3 and Omega-6 ............................... 14 The Beneficial Omega-6 Fatty Acid: GLA ............................................... 16 How Fatty Acids Affect Brain Health ..................................................... 17 Omega-7 Fatty Acids ............................................................................ 18 Monounsaturated Fat ............................................................................ 18 Trans Fats ............................................................................................. 20 Trans Fats and Health ........................................................................... 21 Toxins in Fat .......................................................................................... 22 A Case for Organic ................................................................................ 23 DRHYMAN.COM 3 PART II .......................................... 24 Animal Fats ....................................................................... -

Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products As Sustainable Source of Lipophilic Antioxidants
antioxidants Article Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants Ivana Dimi´c 1, Nemanja Tesli´c 2 , Predrag Putnik 3 , Danijela Bursa´cKovaˇcevi´c 3 , Zoran Zekovi´c 1, Branislav Šoji´c 1, Živan Mrkonji´c 1, Dušica Colovi´cˇ 2 , Domenico Montesano 4,* and Branimir Pavli´c 1,* 1 Faculty of Technology, University of Novi Sad, Blvd. cara Lazara 1, 21000 Novi Sad, Serbia; [email protected] (I.D.); [email protected] (Z.Z.); [email protected] (B.Š.); [email protected] (Ž.M.) 2 Institute of Food Technology, University of Novi Sad, Blvd. cara Lazara 1, 21000 Novi Sad, Serbia; nemanja.teslic@fins.uns.ac.rs (N.T.); dusica.colovic@fins.uns.ac.rs (D.C.)ˇ 3 Faculty of Food Technology and Biotechnology, University of Zagreb, Pierottijeva 6, 10000 Zagreb, Croatia; [email protected] (P.P.); [email protected] (D.B.K.) 4 Section of Food Science and Nutrition, Department of Pharmaceutical Sciences, University of Perugia, Via San Costanzo 1, 06126 Perugia, Italy * Correspondence: [email protected] (D.M.); [email protected] (B.P.) Received: 25 May 2020; Accepted: 23 June 2020; Published: 1 July 2020 Abstract: The aim of this study was to valorize the oil recovery from red and white grape seeds (Vitis vinifera L.) that remains as by-product after the winemaking process. Oils were extracted by modern techniques, ultrasound assisted (UAE), microwave assisted (MAE) and supercritical fluid extraction (SFE), and compared to the Soxhlet extraction (SE). -
Daily Practice
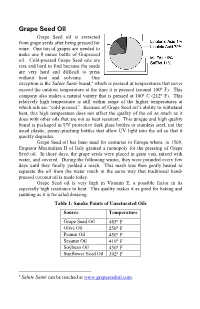
Grape Seed Oil Grape Seed oil is extracted from grape seeds after being pressed for wine. One ton of grapes are needed to make one 8 ounce bottle of Grapeseed oil. Cold-pressed Grape Seed oils are rare and hard to find because the seeds are very hard and difficult to press without heat and solvents. One exception is the Salute Santé brand,a which is pressed at temperatures that never exceed the outdoor temperature at the time it is pressed (around 100° F). This company also makes a natural variety that is pressed at 100° C (212° F). This relatively high temperature is still within range of the higher temperatures at which oils are “cold-pressed.” Because of Grape Seed oil’s ability to withstand heat, this high temperature does not affect the quality of the oil as much as it does with other oils that are not as heat resistant. This unique and high quality brand is packaged in UV protective dark glass bottles or stainless steel, not the usual plastic, penny-pinching bottles that allow UV light into the oil so that it quickly degrades. Grape Seed oil has been used for centuries in Europe where, in 1569, Emperor Maximilian II of Italy granted a monopoly for the pressing of Grape Seed oil. In those days, the grape seeds were placed in giant vats, mixed with water, and covered. During the following winter, they were pounded every few days until they finally yielded a mash. This mash was then gently heated to separate the oil from the water much in the same way that traditional hand- pressed coconut oil is made today. -

Bma Usa Loc 12-5-201
סב '' ד ד ׳ לסכ ו שת פ׳׳ December 5, 2019 To Whom it may concern: This is to certify that the following 64 products: BRAND PRODUCT MASSIMO gusto Extra Virgin Olive Oil MASSIMO gusto Pure Grape Seed Oil MASSIMO gusto Blended Grape Seed Oil MASSIMO gusto Extra Virgin Avocado Oil MASSIMO gusto Extra Virgin Avocado Oil with Garlic MASSIMO gusto Blended Vegetable Oil 75% Canola Oil 25% Extra Virgin Olive Oil MASSIMO gusto Extra Virgin Avocado Oil Blend MASSIMO gusto Balsamic Vinegar of Modena MASSIMO gusto Mediterranean Blend MASSIMO gusto Pomace Oil MASSIMO gusto Pure Olive Oil MASSIMO gusto Pomace Olive Oil MASSIMO gusto Grape Seed Oil MASSIMO gusto Canola Oil MASSIMO gusto Soybean Oil MASSIMO gusto Rice Bran Oil MG Estate Reserve Organic Extra Virgin Olive Oil First Cold Pressed MG Estate Reserve Extra Virgin Avocado Oil MG Estate Reserve Grape Seed Oil MG Pure Grape Seed Oil MG Walnut Oil MG Sweet Almond Oil MG Safflower Oil MG Roasted Sesame Oil MG Flax Seed Oil MG Peanut Oil MG Rice Bran Oil MG Limited Reserve Extra Virgin Olive Oil First Cold Pressed Californian Grown MG Limited Reserve First Cold Pressed Avocado Oil with Garlic MG Organic Extra Virgin Olive Oil Infuse with Lemon MG Organic Extra Virgin Olive Oil Infuse with Lime MG Organic Extra Virgin Olive Oil Infuse with Orange North American Kosher Supervision (Flag K) BMA USA Kosher Certificate 5780 Page 1 of 3 MG Organic Extra Virgin Olive Oil Infused with Jalapeno Pepper MG Organic Extra Virgin Olive Oil Infused with Garlic MG Organic Extra Virgin Olive Oil Infused with Basil -

Evoil Spa Bergamot
EVOIL® SPA BERGAMOT PRODUCT DATA SHEET EVOIL® SPA BERGAMOT is a composition of vegetable oils and bergamot essential oil in order to create a multi- functional oil. Plants elaborate essential oils with the purpose of protecting themselves from diseases, drive away predator insects or attract beneficial insects. Essential oils act, among other ways, by stimulating the sense of smell, harmonizing the psychic and emotional states since smell is directly connected to the zone of the brain where the center of emotions is located. Essential oils by themselves are very powerful concentrates, and unless indicated otherwise, should not be directly applied to the skin or irritation can result. EVOIL® SPA BERGAMOT is formulated in natural vegetable oils: Sweet Almond, Jojoba, Grape seed and Sunflower oils, all of them renowned for their excellent emollient properties. DESCRIPTION OF INGREDIENTS BERGAMOT OIL is the essential oil obtained by steam- distillation from Citrus bergamia, a South East Asia native plant, but then introduced to Europe, and particularly Italy. Its aroma is described as citric, fruity and sweet. Historically, BERGAMOT OIL has been an ingredient in Italian folk remedies for fevers and worms for centuries. BERGAMOT OIL contains about 300 active constituents. This may explain its many beneficial uses. The main active components are -pinene, myrcene, limonene and -bergaptene. The high content in -bergaptene may cause increased photosensitivity when the skin is exposed to sunlight. Another property of BERGAMOT ESSENTIAL OIL is that it has superb antiseptic qualities that are useful for skin disorders, such as acne, oily skin conditions and eczema and it can also be used on cold sores and wounds. -

Food & Chemical Effects on Acid/Alkaline Body Chemical Balance
Food & Chemical Effects on Acid/Alkaline Body Chemical Balance acid forming foods, alkaline forming foods, ph of foods, acid balance, alkalinity, foods high in acid, basic foods, balancing digestion, balancing digestive system, MOST ALKALINE MORE ALKALINE LOW ALKALINE LOWEST ALKALINE FOOD CATEGORY LOWEST ACID LOW ACID MORE ACID MOST ACID Baking Soda Spices / Cinnamon Herbs (most) SPICES / HERBS Curry Vanilla Nutmeg Pudding / Jam / Jelly Sea Salt Sulfite PRESERVATIVES MSG Benzoate Aspartame Table Salt (NaCl) Mineral Water, Herb Kambucha Green or mu tea Ginger Tea BEVERAGES Tea, Kona Coffee Alcohol Coffee Beer Teas, Lemon Water Black Tea Yeast / Hops / Malt, Soft Drinks Soy Sauce Apple Cider Vinegar Umeboshi vinegar VINEGARS Rice Vinegar Balsamic Vinegar White Acid Vinegar Stevia Maple Syrup, Rice Raw Honey, Raw Sugar SWEETENERS Honey/Maple Syrup Stevia Saccharin Sugar / Cocoa Syrup Umeboshi plums Sake Algae, blue-green THERAPEUTICS Antihistamines Psychotropics Antibiotics Lemons, Dates, Figs, Oranges, Bananas, FRUITS Plums, Processed Sour Cherries, Cranberries, Prunes Watermelon, Limes, Melons, Grapes, Cherries, Pineapple, Fruit Juices Rhubarb Grapefruit, Mangoes, Kiwi, Apples, Peaches, Avocados Papayas Pears, Raisins Lentils Kohlrabi Potato / Bell pepper Brussel sprout Spinach Split pea Green pea Soy Bean Brocoflower Parsnip / Taro Mushroom / Fungi Beet BEANS VEGETABLES Fava beans Pinto beans Peanut Carob Garlic Cauliflower Chive / Cilantro LEGUMES Kidney beans White beans Snow pea Seaweed Asparagus Cabbage Celery PULSES Black-eyed peas -

Redalyc.Grape Seed Oil: a Potential Functional Food?
Ciência e Tecnologia de Alimentos ISSN: 0101-2061 [email protected] Sociedade Brasileira de Ciência e Tecnologia de Alimentos Brasil Branco SHINAGAWA, Fernanda; Carvalho de SANTANA, Fernanda; Oliveira TORRES, Lucillia Rabelo; MANCINI-FILHO, Jorge Grape seed oil: a potential functional food? Ciência e Tecnologia de Alimentos, vol. 35, núm. 3, julio-septiembre, 2015, pp. 399-406 Sociedade Brasileira de Ciência e Tecnologia de Alimentos Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=395942248001 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative a Food Science and Technology ISSN 0101-2061 DDOI http://dx.doi.org/10.1590/1678-457X.6826 Grape seed oil: a potential functional food? Fernanda Branco SHINAGAWA1, Fernanda Carvalho de SANTANA1, Lucillia Rabelo Dliveira TDRRES1, Jorge MANCINI-FILHD1* Abstract Grape seed oil (GSD) is not often consumed in Brazil and little is known of its nutritional value. Around the world there are already studies that point to the high levels of minority bioactive compounds and their relation to health benefits. The main constituent of GSD is linoleic fatty acid, some works are controversial and there is no consensus in literature regarding their effect on the animal organism. Thus, this study aimed to present a review of GSD and show the potential health effects of its major components, not only linoleic acid, but also γ-tocotrienol and β-sitosterol, and finally, their influence on lipid-modulating, anti and pro oxidative parameters. -

Essential Fatty Acids (Efas): Your Brain and Body
Essential Fatty Acids (EFAs): Your Brain and Body Fatty acids are the basic building blocks of which fats and oils are composed. Contrary to popular myth, the body does need fat. It must be the right kind, however. The fatty acids that are necessary for health and that cannot be made by the body are called essential fatty acids (EFAs). They are occasionally also referred to as vitamin F or polyunsaturates. EFAs must be supplied through the diet. Essential fatty acids have desirable effects on many disorders. They improve the skin and hair, reduce blood pressure, aid in the prevention of arthritis, lower cholesterol and triglyceride levels, and reduce the risk of blood clot formation. They are beneficial for candidiasis, cardiovascular disease, eczema, and psoriasis. Found in high concentrations in the brain, EFAs aid in the transmission of nerve impulses and are needed for the normal development and functioning of the brain. A deficiency of essential fatty acids can lead to an impaired ability to learn and recall information. Every living cell in the body needs essential fatty acids. They are essential for rebuilding and producing new cells. Essential fatty acids are also used by the body for the production of prostaglandins, hormone like substances that act as chemical messengers and regulators of various body processes. There are two basic categories of essential fatty acids, designated omega-3 and omega-6, based on their chemical structures. Omega-3 EFAs, including alpha-linolenic and eicosapentaenoic acid (EPA), are found in fresh deepwater fish, fish oil, and certain vegetable oils, and walnut oil. -

INCORPORATING GRAPE SEED ANTIOXIDANTS INTO a FUNCTIONAL FOOD MODEL Lena Binzer Rebecca Brinsko Je
ABSTRACT Title of Document: INCORPORATING GRAPE SEED ANTIOXIDANTS INTO A FUNCTIONAL FOOD MODEL Lena Binzer Rebecca Brinsko Jessica Cha Zao Chen Sarah Green Kelly Grob Junjie Hao Christina Hitz Laura Li Sowmya Swamy Maxim Wolf MengMeng Xu Mary Yanik Directed By: Dr. Liangli Yu, PhD, Department of Nutrition and Food Science Dr. Margaret Slavin, PhD, RD, Department of Nutrition and Food Science Consumption of foods rich in natural antioxidants may potentially reduce the risk of chronic illnesses. This study examined feasibility and consumer acceptability of creating a functional food rich in natural antioxidants from cold-pressed grape seed oil and flour. The first study investigated and compared five grape seed varieties, and found Chardonnay grape seeds contained the highest quantity of health-beneficial properties. Consequently, addition of Chardonnay grape seed flour and oil to bread significantly increased health-beneficial properties. Baking conditions influenced antioxidant properties of bread, indicating processing conditions may affect antioxidant activity in finished food products. In addition, the consumer sensory evaluation study found control bread was preferred over bread containing grape seed flour and oil; however, grape seed containing bread had an overall positive reception. Incorporation of grape seeds into bread may be effective in incorporating health-beneficial compounds into the diet; however, further studies on long term health effects should be conducted. INCORPORATING GRAPE SEED ANTIOXIDANTS INTO A FUNCTIONAL FOOD MODEL By Team Innovative Medicines for Maladies Utilizing Nutraceutical Enhancements (IMMUNE) Lena Binzer, Rebecca Brinsko, Jessica Cha, Zao Chen, Sarah Green, Kelly Grob, Junjie Hao, Christina Hitz, Laura Li, Sowmya Swamy, Maxim Wolf, MengMeng Xu, & Mary Yanik Thesis submitted in partial fulfillment of the requirements for the Gemstone Program, University of Maryland, College Park 2011 Advisory Committee: Dr. -

Antimicrobial Effect of Grape Seed Oil, Olive Oil, and Sesame Oil on Food Poisoning Bacteria Isolated from Raw Meat
1 Plant Archives Vol. 20, Supplement 2, 2020 pp. 4066-4068 e-ISSN:2581-6063 (online), ISSN:0972-5210 ANTIMICROBIAL EFFECT OF GRAPE SEED OIL, OLIVE OIL , AND SESAME OIL ON FOOD POISONING BACTERIA ISOLATED FROM RAW MEAT Aseel, M.H. Abed AL-Rhada Department of Public Health, Veterinary College, Baghdad University, Iraq Abstract The study designed to explore the Antimicrobial effect of the Grape Seed oil, olive oil and Sesame oil against E. coli, E. coli O157, Salmonella, Pseudomonas, Klebsiella, Campylobacter and Yersinia enteriocolitica isolated from Raw meat samples collected from local butchers market in Baghdad. Microorganisms were planted on Muller Hinton agar. The oils were applied using a steers replicator, then incubated at (37˚C) for 24 hours. The antibacterial activities were determined by measuring the inhibition zone diameter in mm. The result showed that biggest inhibition zones were seen with Sesame oil on all bacteria isolate followed by Grape Seed oil then olive oil. Keyword : Grape Seed oil , olive oil, garlic. Introduction Salmonella, Pseudomonas, Klebsiella, Campylobacter and ersinia enteriocolitica Food borne bacteria had constantly been as a most Y . important cause of serious humans diseases. In the last years 25 gram of each meat sample was inoculated into (225) many pharmaceutical companies produced new antibacterial ml of peptone broth and incubated at (37°C) for (18–24) drugs, but, the appearance of bacterial strains with multiple hours. After incubation, about 100µl of the inoculated resistances will struggle these drugs. Furthermore, the wide peptone broth were sub-cultured onto plates of blood agar, use of immune-suppressing drugs with a rise in bacterial Nutrient agar, MacConkey agar, Eosin methylene blue infections become a global concern. -

Resources Refined Vs. Unrefined Oils Cooking with Butter, Lard Or Ghee
Refined vs. unrefined oils Vegetable shortening, ALL ABOUT As with flours or sweeteners, oils can be refined margarine and partially- hydrogenated oils (trans fats) by a process that removes the “impurities” that Oils give an oil its naturally occurring flavor, color In order to stabilize oils and preserve their and nutrients. In exchange, the oil becomes shelf life, food scientists developed a method stable at higher temperatures. Refined oil is not called “hydrogenation” which adds hydrogen StrongerTogether.coop is a consumer website necessarily a bad thing. Many types of foods we to the molecular structure of vegetable oils. developed by National Co+op Grocers (NCG) for like to eat are cooked at high temperatures and This renders oils solid at room temperature our “virtual chain” of over 140 retail food co-ops, require oils that can safely withstand high heat. (vegetable shortening is an example). This operating more than 190 storefronts, nationwide. For low- or no-heat applications, unrefined oils process produces “trans fats.” According to are superior since they contribute flavor and the U.S. Food and Drug Administration (FDA), StrongerTogether.coop is a place for people to nutrients essential to a healthy diet. trans fat raises low-density lipoprotein (LDL) gather on their food journeys. It’s a place to find out or “bad cholesterol” in the blood and increases more about what’s in your food, where it comes Cooking with butter, lard or the risk of heart disease. For many years, from, where to find great food, how to prepare it ghee trans fats have been the primary choice for and a whole lot more. -

(19) United States (12) Patent Application Publication (10) Pub
US 20070178061A1 (19) United States (12) Patent Application Publication (10) Pub. N0.: US 2007/0178061 A1 Venturi et a]. (43) Pub. Date: Aug. 2, 2007 (54) THERAPEUTIC BLENDED OIL (52) US. Cl. .......................... .. 424/74; 424/735; 424/757; COMPOSITION AND METHOD 424/766; 424/769 (76) Inventors: Marco Venturi, Lake BluiT, IL (US); Tara Venturi-Jackson, Lake BluiT, IL (Us) (57) ABSTRACT Correspondence Address: GERRY FISCHER 954 ARNOLD COURT . DES PLAINES, IL 60016 (Us) A therapeut1c blended oil compos1t1on 1s provided that~1s useful as a topical applicatlon to be absorbed into skin, (21) Appl. No.: 11/346,033 protect the skin from sun Without inducing acne, reduce skin _ Wrinkles lines and dryness. The therapeutic oil composition (22) F1led: Feb. 2, 2006 is. created by m1x1ng. grape seed o1l,. soy bean o1l,. sWeet Publication Classi?cation almond oil, sea buckthom seed oil and essential oils. The volume of each constituent added can be modi?ed according (51) Int‘ C1‘ to the desired treatment. The composition may be applied A61K 8/97 (200601) to icall to absorb into the skin to aid skin tone tannin A61K 36/87 (2006.01) P_ X ’ _ _ g’ A 61K 36/48 (200601) moisturizing, eczema, and burn symptoms. The compos1t1on A61]; 36/736 (200601) is light oil that does not leave the skin feeling oily and is an A61K 36/72 (2006.01) effective and safe makeup remover. US 2007/0178061 A1 Aug. 2, 2007 THERAPEUTIC BLENDED OIL COMPOSITION nutrients contained in grape seed oil. The temperature is kept AND METHOD loW to preserve the valuable nutrients of the grape seed oil.