Thymelaea Microphylla Andgnidia Somalensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plants for a Future Species Database Bibliography
Plants For A Future Species Database Bibliography Numbers in square brackets are the reference numbers that appear in the database. [K] Ken Fern Notes from observations, tasting etc at Plants For A Future and on field trips. [1] F. Chittendon. RHS Dictionary of Plants plus Supplement. 1956 Oxford University Press 1951 Comprehensive listing of species and how to grow them. Somewhat outdated, it has been replaces in 1992 by a new dictionary (see [200]). [1b] Food Plants International. http://foodplantsinternational.com/plants/ [1c] Natural Resources Conservation Service http://plants.usda.gov [1d] Invasive Species Compendium www.cabi.org [2] Hedrick. U. P. Sturtevant's Edible Plants of the World. Dover Publications 1972 ISBN 0-486-20459-6 Lots of entries, quite a lot of information in most entries and references. [3] Simmons. A. E. Growing Unusual Fruit. David and Charles 1972 ISBN 0-7153-5531-7 A very readable book with information on about 100 species that can be grown in Britain (some in greenhouses) and details on how to grow and use them. [4] Grieve. A Modern Herbal. Penguin 1984 ISBN 0-14-046-440-9 Not so modern (1930's?) but lots of information, mainly temperate plants. [5] Mabey. R. Food for Free. Collins 1974 ISBN 0-00-219060-5 Edible wild plants found in Britain. Fairly comprehensive, very few pictures and rather optimistic on the desirability of some of the plants. [6] Mabey. R. Plants with a Purpose. Fontana 1979 ISBN 0-00-635555-2 Details on some of the useful wild plants of Britain. Poor on pictures but otherwise very good. -

APPROVED PLANT LIST Midtown Alliance Tree Well Adoption Program
APPROVED PLANT LIST Midtown Alliance Tree Well Adoption Program Midtown Alliance launched the Tree Well Adoption program with the primary goal of enriching the experience of Midtown’s workers and residents while encouraging sustainability through the use of low-water, urban tolerant plant species. This list of plants was created to aid individuals and organizations in selecting plant material to plant in their adopted tree wells. This plant list is intended to encourage individual character in the tree wells, rather than restrict creativity in the selection of plants. The plants on the approved list were selected based on the following criteria: • Perennial. All plants listed are perennial, meaning they last for two or more growing seasons. Once established, these plants will require less water to maintain than annuals. • Heat tolerant. Plants in tree wells are exposed to high temperatures caused by vehicles and heat reflected from surrounding buildings, asphalt, and other urban surfaces. They must also be tolerant to high daytime temperatures, typical of Atlanta’s summer months, and cold hardy in the winter months. Atlanta is located in USDA Plant Hardiness Zone 7b/8a. • Water wise. Urban tree wells are surrounded by impervious surfaces and thus, are highly susceptible to periods of drought. Suitable plants must be able to survive periods of low rainfall. • Pollution tolerant. Vehicle exhaust may leave deposits and pollutants on plant foliage, which can kill sensitive plants. • Encourage wildlife. Flowering plants attract insects such as butterflies while others provide food sources for birds and other wildlife. • Grown locally. Many of the plants listed are native to the Atlanta area, and all can be found at local nurseries. -

Pdf (306.01 K)
REVIEW ARTICLE RECORDS OF PHARMACEUTICAL AND BIOMEDICAL SCIENCES Review article on chemical constituents and biological activity of Thymelaea hirsuta. Ahmed M Badawya, Hashem A Hassaneanb, Amany K. Ibrahimb, Eman S. Habibb, Safwat A. Ahmedb* aDepartment of Pharmacognosy, Faculty of Pharmacy, Sinai University, El-Arish, Egypt, b Department of Pharmacognosy, Faculty of Pharmacy, Suez Canal University, Ismailia, Egypt 41522. Abstract Received on: 07.04. 2019 Thymelaea hirsuta a perennial, evergreen and dioecious shrub, which is native Revised on: 30. 04. 2019 to North Africa. T. hirsuta is a widespread invasive weed and is commonly known as “Methnane”. Along the history, T. hirsuta, family Thymelaeaceae, Accepted on: 10. 04. 2019 has been recognized as an important medicinal plant. Much research has been carried out on the medical applications of Methnane. The choice of the plant was based on the good previous biological study of T. hirsuta plant extract to Correspondence Author: use as anticancer, hepatoprotective and anti-diapetic. Several species of Tel:+ 01092638387 Thymelaeaceae have been the subject of numerous phytochemical studies. Initially, interest may have been due to the marked toxicity of these plants, but E-mail address: the widespread use of some species medicinally has certainly played a part in [email protected] sustaining this interest. Keywords: Thymelaea hirsuta , Chemical constituents, Biological activity 1.Introduction: Near East: Lebanon and Palestine. The choice of the plant was based on the good previous Thymelaea hirsuta a perennial, evergreen and biological study of T. hirsuta plant extract to use dioecious shrub, which is native to North Africa. T. as anticancer, hepatoprotective and anti-diabetic. -

Thymelaeaceae)
Origin and diversification of the Australasian genera Pimelea and Thecanthes (Thymelaeaceae) by MOLEBOHENG CYNTHIA MOTS! Thesis submitted in fulfilment of the requirements for the degree PHILOSOPHIAE DOCTOR in BOTANY in the FACULTY OF SCIENCE at the UNIVERSITY OF JOHANNESBURG Supervisor: Dr Michelle van der Bank Co-supervisors: Dr Barbara L. Rye Dr Vincent Savolainen JUNE 2009 AFFIDAVIT: MASTER'S AND DOCTORAL STUDENTS TO WHOM IT MAY CONCERN This serves to confirm that I Moleboheng_Cynthia Motsi Full Name(s) and Surname ID Number 7808020422084 Student number 920108362 enrolled for the Qualification PhD Faculty _Science Herewith declare that my academic work is in line with the Plagiarism Policy of the University of Johannesburg which I am familiar. I further declare that the work presented in the thesis (minor dissertation/dissertation/thesis) is authentic and original unless clearly indicated otherwise and in such instances full reference to the source is acknowledged and I do not pretend to receive any credit for such acknowledged quotations, and that there is no copyright infringement in my work. I declare that no unethical research practices were used or material gained through dishonesty. I understand that plagiarism is a serious offence and that should I contravene the Plagiarism Policy notwithstanding signing this affidavit, I may be found guilty of a serious criminal offence (perjury) that would amongst other consequences compel the UJ to inform all other tertiary institutions of the offence and to issue a corresponding certificate of reprehensible academic conduct to whomever request such a certificate from the institution. Signed at _Johannesburg on this 31 of _July 2009 Signature Print name Moleboheng_Cynthia Motsi STAMP COMMISSIONER OF OATHS Affidavit certified by a Commissioner of Oaths This affidavit cordons with the requirements of the JUSTICES OF THE PEACE AND COMMISSIONERS OF OATHS ACT 16 OF 1963 and the applicable Regulations published in the GG GNR 1258 of 21 July 1972; GN 903 of 10 July 1998; GN 109 of 2 February 2001 as amended. -

Annual Report
Darwin Initiative Annual Report 1. Darwin Project Information Project Ref. Number 14-013 Project Title Community Management of NTFPs in Kangchenjunga Conservation Area, Nepal Country(ies) Nepal UK Contractor WWF UK Partner Organisation(s) WWF Nepal Program Darwin Grant Value GBP 170,100 Start/End dates April 2005-March 2008 Reporting period and 1 April 2005-31 March 2006 annual report number Annual report 1 Project website Author(s), date Suresh K. Ghimire, Bal Krishna Nepal, Parag Bijukchhe, Yeshi Lama, Jennifer Headley & Ros Coles 23 May 2006 2. Project Background • Briefly describe the location and circumstances of the project and the problem that the project aims to address. Situated in the remote, north-east corner of Nepal, bordering Sikkim (India) and Tibet Autonomous Region (China), Kangchenjunga Conservation Area (KCA) is characterized by great variations in elevation, climate, landscape, habitat, vegetation, and cultural diversity. Phyto-geographically, it forms the meeting ground of the Indo- Malaysian (S.E. Asian) and Sino-Japanese (E. Asian) floras. KCA has been declared as a “Gift to the Earth” in 1997. In 1998, WWF initiated the Kangchenjunga Conservation Area Project (KCAP) to conserve its rich biodiversity and promote community-based management of natural resources. About 1500 species of flowering plants have been documented thus far in KCA (Shrestha and Ghimire 1996), including over 150 species of non-timber forest products (Shrestha and Ghimire 1996; Sherpa 2001, Oli and Nepal 2003). NTFPs play an important role in supporting the livelihood of about 5000 people living in the four village development committees (VDCs) of Lelep, Tapethok, Yamphudin, and Walangchung Gola. -

GENERA REPRESENTED in THIS NUMBER Acer Amygdalus
GENERA REPRESENTED IN THIS NUMBER Page Page Acer 1925 Edgeworthia 1922 Amygdalus 1926, 1927 Gladiolus 1922 Buddleia 1921, 1927 Haematoxylum 1927 Cassia 1927 Musa 1922 Chamaedorea 1927 Quercus 1922 Citrus 1921 Veronica 1923 Crotalaria 1921, 1922 Notes and Comments The Favorita pear (S. P. I. No. 33207). 339. The Chilean strawberry as marketed in Colombia (Fragaria chiloensis). 340. A nitrogen * gathering shrub from the Comoro Islands {Psychotria bacteriophila). EXPLANATORY NOTE PLANT IMMIGRANTS is designed principally to call the attention of plant breeders and experimenters to the arrival of interesting plant material. It should not be viewed as an announcement of plants available for distribution, since most introductions have to be propagated before they can be sent to experimenters. This requires from one to three years, depending upon the nature of the plant and the quantity of live material received. As rapidly as stocks are available, the plants described in this circular will be included in the Annual List of Plant Introductions, which is sent to experimenters in late autumn. Introductions made for a special purpose (as for example to supply Department and other specialists with material needed in their experiments) are not propagated by this Office and will not appear in the Annual List. Descriptions appearing here are revised and later published in the Inventory of Seed,s and Plants Imported, -the permanent record of plant in- troductions made by this Office. Plant breeders and experimenters who desire plants not available in this country are invited to correspond with this Office which will endeavor to secure the required material through its agricultural ex- plorers, foreign collaborators, or correspondents. -

Dil Limbu.Pmd
Nepal Journal of Science and Technology Vol. 13, No. 2 (2012) 87-96 A Checklist of Angiospermic Flora of Tinjure-Milke-Jaljale, Eastern Nepal Dilkumar Limbu1, Madan Koirala2 and Zhanhuan Shang3 1Central Campus of Technology Tribhuvan University, Hattisar, Dharan 2Central Department of Environmental Science Tribhuvan University, Kirtipur, Kathmandu 3International Centre for Tibetan Plateau Ecosystem Management Lanzhou University, China e-mail:[email protected] Abstract Tinjure–Milke–Jaljale (TMJ) area, the largest Rhododendron arboreum forest in the world, an emerging tourist area and located North-East part of Nepal. A total of 326 species belonging to 83 families and 219 genera of angiospermic plants have been documented from this area. The largest families are Ericaceae (36 species) and Asteraceae (22 genera). Similarly, the largest and dominant genus was Rhododendron (26 species) in the area. There were 178 herbs, 67 shrubs, 62 trees, 15 climbers and other 4 species of sub-alpine and temperate plants. The paper has attempted to list the plants with their habits and habitats. Key words: alpine, angiospermic flora, conservation, rhododendron Tinjure-Milke-Jaljale Introduction determines overall biodiversity and development The area of Tinjure-Milke-Jaljale (TMJ) falls under the activities. With the increasing altitude, temperature middle Himalaya ranging from 1700 m asl to 5000 m asl, is decreased and consequently different climatic and geographically lies between 2706’57" to 27030’28" zones within a sort vertical distance are found. The north latitude and 87019’46" to 87038’14" east precipitation varies from 1000 to 2400 mm, and the 2 longitude. It covers an area of more than 585 km of average is about 1650 mm over the TMJ region. -

Islet Protection and Amelioration of Type 2 Diabetes Mellitus by Treatment with Quercetin from the Flowers Ofedgeworthia Gardneri
Journal name: Drug Design, Development and Therapy Article Designation: Original Research Year: 2018 Volume: 12 Drug Design, Development and Therapy Dovepress Running head verso: Zhuang et al Running head recto: Pharmacological activities of quercetin from the flowers of Edgeworthia gardneri open access to scientific and medical research DOI: 153898 Open Access Full Text Article ORIGINAL RESEARCH Islet protection and amelioration of type 2 diabetes mellitus by treatment with quercetin from the flowers of Edgeworthia gardneri Manjiao Zhuang1 Background and purpose: The traditional Chinese medicine – the flower of Edgeworthia Honghong Qiu2 gardneri – is reported as an effective therapeutic for type 2 diabetes mellitus (T2DM). Ping Li2 Nevertheless, most constituents of the flowers of E. gardneri have not yet been stud- Lihua Hu2 ied. This study was conducted to investigate the effect of quercetin extracted from the Yayu Wang3 flowers of E. gardneri on islet protection and amelioration in T2DM and explore its Lei Rao2 mechanism. Method: Quercetin was extracted from the flowers of E. gardneri and verified by high-per- 1 Department of Pharmacy, Sichuan formance liquid chromatography. Quercetin or crude extract’s effect on insulin secretion was University, Chengdu, People’s Republic of China; 2Collaborative Innovation investigated. ERK1/2 and phospho-ERK1/2 were detected by Western blot analysis, and fluo-3 Center of Sichuan for Elderly Care AM was used to detect intracellular Ca2+. The anti-apoptosis effect of quercetin or crude extract and Health, Chengdu Medical College, on MIN-6 cells was investigated by thiazolyl blue tetrazolium bromide (MTT) assay and flow Chengdu, China; 3Department of Cell Biology, Institute of Biological cytometry analysis. -
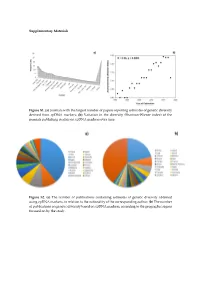
(A) Journals with the Largest Number of Papers Reporting Estimates Of
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Phytochemistry of Dais Cotinifolia L
Phytochemistry of Dais cotinifolia L. Thesis submitted in fulfilment of the requirements for the degree Master of Science By Busisiwe Buyisile Danca School of Chemistry and Physics University of KwaZulu-Natal Pietermaritzburg Supervisor: Professor Fanie R. van Heerden May 2018 | Page Abstract Many plant-derived secondary metabolites have interesting biological activities and some plant compounds such as artemisinin (antimalarial) and vincristine (anticancer agent) are the active principles of main-stream pharmaceuticals. South Africa has an immense biodiversity and there are in the order of 25 000 indigenous plant species in the country. The phytochemistry of many of these plants has never been investigated. Many bioactive compounds have been isolated from the Thymelaeaceae, such as prostatin (potent anti-HIV agent) and antitumour diterpenoids. Although a large number of Thymelaeaceae species are indigenous to South Africa, only a limited number of species have been subjected to phytochemical investigations. Dais cotinifolia L. is one species on which phytochemical results have not been published and this tree was the subject of this investigation. Four compounds were isolated from D. cotinifolia. Three of these compounds are known metabolites, two furofuran lignans, kobusin and eudesmin, and the flavonoid catechin. A fourth compound is novel norlignan, 2-hydroxy-5-(3-methoxyphenyl)-1-(4- methoxyphenyl)pentan-1-one. The compounds were isolated by applying different chromatographic procedures, such as column chromatography, preparative centrifugal thin-layer chromatography and semi-preparative high-performance liquid chromatography. The structural elucidation of the compounds was based on mass spectrometry and nuclear magnetic spectroscopy. The antioxidant activities of the crude extracts of the different plant parts were determined by the DPPH assay. -

Heterodichogamy.Pdf
Research Update TRENDS in Ecology & Evolution Vol.16 No.11 November 2001 595 How common is heterodichogamy? Susanne S. Renner The sexual systems of plants usually Heterodichogamy differs from normal (Zingiberales). These figures probably depend on the exact spatial distribution of dichogamy, the temporal separation of underestimate the frequency of the gamete-producing structures. Less well male and female function in flowers, in heterodichogamy. First, the phenomenon known is how the exact timing of male and that it involves two genetic morphs that is discovered only if flower behavior is female function might influence plant occur at a 1:1 ratio. The phenomenon was studied in several individuals and in mating. New papers by Li et al. on a group discovered in walnuts and hazelnuts5,6 natural populations. Differential of tropical gingers describe differential (the latter ending a series of Letters to movements and maturation of petals, maturing of male and female structures, the Editor about hazel flowering that styles, stigmas and stamens become such that half the individuals of a began in Nature in 1870), but has gone invisible in dried herbarium material, population are in the female stage when almost unnoticed7. Indeed, its recent and planted populations deriving from the other half is in the male stage. This discovery in Alpinia was greeted as a vegetatively propagated material no new case of heterodichogamy is unique new mechanism, differing ‘from other longer reflect natural morph ratios. The in involving reciprocal movement of the passive outbreeding devices, such as discovery of heterodichogamy thus styles in the two temporal morphs. dichogamy…and heterostyly in that it depends on field observations. -

Towards an Updated Checklist of the Libyan Flora
Towards an updated checklist of the Libyan flora Article Published Version Creative Commons: Attribution 3.0 (CC-BY) Open access Gawhari, A. M. H., Jury, S. L. and Culham, A. (2018) Towards an updated checklist of the Libyan flora. Phytotaxa, 338 (1). pp. 1-16. ISSN 1179-3155 doi: https://doi.org/10.11646/phytotaxa.338.1.1 Available at http://centaur.reading.ac.uk/76559/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.11646/phytotaxa.338.1.1 Identification Number/DOI: https://doi.org/10.11646/phytotaxa.338.1.1 <https://doi.org/10.11646/phytotaxa.338.1.1> Publisher: Magnolia Press All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Phytotaxa 338 (1): 001–016 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.338.1.1 Towards an updated checklist of the Libyan flora AHMED M. H. GAWHARI1, 2, STEPHEN L. JURY 2 & ALASTAIR CULHAM 2 1 Botany Department, Cyrenaica Herbarium, Faculty of Sciences, University of Benghazi, Benghazi, Libya E-mail: [email protected] 2 University of Reading Herbarium, The Harborne Building, School of Biological Sciences, University of Reading, Whiteknights, Read- ing, RG6 6AS, U.K.