Stellera Chamaejasme L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
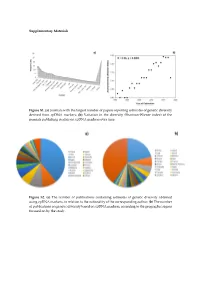
(A) Journals with the Largest Number of Papers Reporting Estimates Of
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Flora of China 13: 248–250. 2007. 8. DIARTHRON Turczaninow, Bull
Flora of China 13: 248–250. 2007. 8. DIARTHRON Turczaninow, Bull. Soc. Imp. Naturalistes Moscou 5: 204. 1832. 草瑞香属 cao rui xiang shu Wang Yinzheng (王印政); Michael G. Gilbert Stelleropsis Pobedimova. Annual or perennial herbs, or small deciduous shrubs. Leaves alternate; leaf blade elliptic, linear, or lanceolate, herbaceous. In- florescence usually terminal, sometimes apparently axillary, laxly racemose to capitate, without involucre. Flowers bisexual, small, 4(or 5)-merous. Calyx reddish, white, or green; tube persistent, slender, urceolate, funnel-shaped, or cylindric, contracted and articu- late at apex of ovary; lobes 4, erect and slightly spreading. Petaloid appendages absent. Stamens as many as or twice as many as calyx lobes, in one or two series, opposite to calyx lobes when in one series; filaments absent; anthers oblong, included. Disk annu- lar, oblique, sometimes tiny or absent. Ovary ± stipitate, glabrous, 1-loculed; style excentric or subterminal, short; stigma subclavate, thick. Fruit dry, enclosed by persistent calyx base; pericarp thin, glossy black. Sixteen species: C and SW Asia, SE Europe (European Russia); four species in China. The authors have accepted the view of Kit Tan (Notes Roy. Bot. Gard. Edinburgh 40: 219–220. 1982) that the difference in habit used to separ- ate the annual herbs of Diarthron sensu stricto from the perennial herbs traditionally placed in Stelleropsis and the small shrubs in the C Asian genus Dendrostellera (C. A. Meyer) Tieghem are outweighed by the similar 4-merous flowers and ebracteate inflorescences combined with the continuous range of variation in habit. 1a. Calyx tube 2–4 mm; annual herbs, mainly branched well above base, roots not thickened; hypogynous disk very reduced or absent; inflorescence terminal, lax, elongated, spikelike. -

Paper Plants in the Tibetan World: a Preliminary Study
CHAPTER 22 Paper Plants in the Tibetan World: A Preliminary Study Alessandro Boesi Tibetan texts, notably religious ones (dpe cha), were inspired by Indian Buddhist manuscripts, which were produced from palm leaves,1 and rep- resented one of the oldest tools of writing in India, and several other Asian Countries as Thailand, Burma, Nepal, and Sri Lanka. Called grantha or later pustaka, they were manufactured in the so-called pothi format – loose long rectangular leaves held together by a string that runs through holes in the mid- dle and then bound around with two covers, usually made of wood. Tibetan manuscripts and printed texts mostly conserve the Indian pothi format. In the seventh century, when Tibet became a powerful empire in Asia and the basis of Tibetan civilization were laid, recording of information became a crucial undertaking, primarily for administrative and ritual purposes. A new script based on a Sanskrit alphabet was devised; stone inscriptions were carved and wood was often used as writing surface but had significant limitations. As paper, and techniques for making it, were imported from neighbouring coun- tries much more extensive writing became possible. It was most likely during this period that Tibetans selected the local plants that could be used to pro- duce paper, and started this activity in their own country. The aim of this article is to examine paper plants in the Tibetan cultural area, particularly focusing on their identification. This will be done by exam- ining accounts from pre-modern Tibet, my field data, research data, and the information available on Tibetan medical treatises. -

Index to the Linnean Herbarium : with Indication of the Types of Species Marked by Carl Von Linné
wm^mmm r'^ .M' 9'* ^T '.^^^H. *'-"•! .^.v^^-^^v wv^^U /<<^-^r'^ :««.r k-.''*a,-V» ;' California Academy of Sciences Library By action of the Board of Trustees of the Leland Stanford Junior University on June 14, 1974, this book has been placed on deposit with the California Academy of Sciences Library. EHHR ,;-**V Jt r V INDEX TO THE LINNEAN HERBARIUM, WITH INDICATION OF THE TYPES OF SPECIES MARKED BY CARL VON LINNE. BY BENJAMIN DAYDON JACKSON, Knight op the Royal Swedish Order of the Polar Star, Hon. Ph.D., & A.M., Upsal. ; General Secretary of the Linnean Society of London. Forming a Supplement to the Proceedings of the Society for the 124th Session, 1911-12. LONDON: PRINTED FOR THE LINNEAN SOCIETY, BURLINGTON HOUSE, PICCADILLY, W., BY TAYLOR AND TRANCIS, RED LION COURT, FLEET STREET. 1912 732377 CONTENTS. Page lutroduction 5 The Linnean Herbarium 7 Plan of Present Index 8 Earlier Enumerations, 1753-1767 8 List of Contributors to the Herbarium 9 Linne as a Collector 18 Signs used in the Herbarium 19 Numbers employed 20 Damage to Herbarium before 1783 21 Collateral Type-collections 21 Bibliography 22 Abbreviations and Signs used in Index 2o Index of specimens in the Linnean Herbarium .... 27 INTEODUCTION. In the autumn o£ 1906 a suggestion was made to the Council of the Linnean Society of London, that a Catalogue of the contents of the Linnean Herbarium, together with a series of photographic illustrations of selected types from it, would he an appropriate publication for the celebration of the 200th anniversary of the birth of Carl von Linne. -

New Invaders of the Northwest, 2Nd Edition
United States TECHNOLOGY Department of Agriculture TRANSFER NEW INVADERS OF THE NORTHWEST Second Edition Rachel Winston, Wendy DesCamp, Jennifer Andreas, Carol Bell Randall, Joseph Milan, and Mark Schwarzländer Forest Health Technology University of Idaho FHTET-2014-12 Enterprise Team Extension December 2014 he Forest Health Technology Enterprise Team (FHTET) was created in T1995 by the Deputy Chief for State and Private Forestry, USDA, Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ Cover photos (clockwise from top left): crown vetch (Dan Tenaglia, Missouriplants.com, www.bugwood. org), garden loosestrife (King County NWCB), giant reed (Amy Ferriter, www.bugwood.org), blueweed (H. Zell), English holly (Jürgen Howaldt) The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, D.C. 20250-9410, or call 202- 720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. -

Tori in Species of Diarthron, Stellera and Thymelaea (Thymelaeaceae)
IAWA54 Journal, Vol. 32 (1), 2011: 54–66 IAWA Journal, Vol. 32 (1), 2011 TORI IN SPECIES OF DIARTHRON, STELLERA AND THYMELAEA (THYMELAEACEAE) Roland Dute*, M. Daniel Jandrlich, Shutnee Thornton, Nicholas Callahan and Curtis J. Hansen Department of Biological Sciences, Auburn University, Life Sciences Building, Auburn, Alabama 36849-5407, U.S.A. * Corresponding author [E-mail: [email protected]] SUMMARY Torus thickenings have been found previously in intervascular pit mem- branes of species of Daphne and Wikstroemia (Thymelaeaceae). A search for tori was undertaken in the closely related genera Diarthron, Stel- lera and Thymelaea. Tori were observed in five of the seven species of Diarthron that were investigated. Presence of tori was associated with commonly occurring imperforate conducting elements and with perennial growth habit. Tori of a different morphology from that of Diarthron were present in two of the three specimens of Stellera chamaejasme that were studied. This study suggests torus presence to have systematic value; spe- cifically, tori are present in species of the subgeneraDendrostellera and Stelleropsis within Diarthron but absent in the subgenus Diarthron. Of 19 species of Thymelaea investigated, only two of four specimens of T. vil- losa contained torus-bearing pit membranes. It is suggested that the origi- nal classification of this species asDaphne villosa be reconsidered. Key words: Diarthron, Stellera, Thymelaea, pit membrane, torus. INTRODUCTION A torus is a centrally located thickening in intervascular pit membranes of wood. Once thought to be rare in angiosperm woods, tori are now known from close to 80 species of eudicots (Dute et al. 2010). Ohtani and Ishida (1978) first discovered tori in three species of Daphne within the Thymelaeaceae. -

Downloaded from Brill.Com09/29/2021 01:43:26PM Via Free Access 502 Boesi Regions and Places Have Represented the Main Centres of Paper Production
CHAPTER 22 Paper Plants in the Tibetan World: A Preliminary Study Alessandro Boesi Tibetan texts, notably religious ones (dpe cha), were inspired by Indian Buddhist manuscripts, which were produced from palm leaves,1 and rep- resented one of the oldest tools of writing in India, and several other Asian Countries as Thailand, Burma, Nepal, and Sri Lanka. Called grantha or later pustaka, they were manufactured in the so-called pothi format – loose long rectangular leaves held together by a string that runs through holes in the mid- dle and then bound around with two covers, usually made of wood. Tibetan manuscripts and printed texts mostly conserve the Indian pothi format. In the seventh century, when Tibet became a powerful empire in Asia and the basis of Tibetan civilization were laid, recording of information became a crucial undertaking, primarily for administrative and ritual purposes. A new script based on a Sanskrit alphabet was devised; stone inscriptions were carved and wood was often used as writing surface but had significant limitations. As paper, and techniques for making it, were imported from neighbouring coun- tries much more extensive writing became possible. It was most likely during this period that Tibetans selected the local plants that could be used to pro- duce paper, and started this activity in their own country. The aim of this article is to examine paper plants in the Tibetan cultural area, particularly focusing on their identification. This will be done by exam- ining accounts from pre-modern Tibet, my field data, research data, and the information available on Tibetan medical treatises. -

Djvu Document
BULL. BOT. SURV. INDIA VoL 21, Nos. 1-4 : pp. 35-67, 1979 COMMEMORATIVE GENERIC NAMES OF FLOWERING PLANTS IN INDIA Botanical Surq of India, Howrah ABSTRACT Commemorative generic names of plants are given in honour of well known botanists and patrons of science for the contributions they made for the cause of botany. The names are given in alphabetical order with their families and one example under each gcnw. A brief biographical sketch of the person is also given. Plants named after botanists and eminent persons form one continuous historical sequence in the development of Natural history. INTRODUCTION In 1867 Alphonse de Candolle, son of A. P. Naming of plants and animals using two- De Candolle with his legal background first word names is as old as human civilization proposed the rules of nomenclature in "Lois and is commonly seen in all languages. In de la Nomenclature Botanique" (1867). In I 753 Carl ~innaeusin his Species Plantarum the Vienna botanical congress (Igo j), these consistently used the two-word system in rules were officially designated as "Interna- Latin for about 5900 Linnean specific epi- tional Rules of Botanical Nomenclature" and thets which was hailed as the binomial since 1952 it is called as "International Code system of nomenclature. In the 18th of Botanical Nomenclature" Plant names century in Europe, Latin was the accepted named after botanists and eminent persous language of scholarship and common form one continuous historical sequence in medium of communication cutting across the development of Natural history. Gen- national boundaries. According to the eric names are given in alphabetical order rules of naming mostly botanical generic and under the first genus Abelia R. -

Effects of Stellera Chamaejasme Removal on the Nutrient Stoichiometry of S
Effects of Stellera chamaejasme removal on the nutrient stoichiometry of S. chamaejasme-dominated grasslands in the Qinghai–Tibetan plateau Meiling Song1, Yuqin Wang1, Gensheng Bao1, Hongsheng Wang1, Yali Yin1, Xiuzhang Li1,2 and Chunping Zhang1 1 Qinghai Academy of Animal and Veterinary Sciences, State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining, Qinghai, China 2 State Key Laboratory of Grassland Agro-Ecosystems, SKLGAE, Lanzhou University, Lanzhou, Gansu, China ABSTRACT Background: Stoichiometric relations drive powerful constraints in several fundamental ecosystem processes. However, limited studies have been conducted on the ecological stoichiometry of plants after the change of community composition induced by Stellera chamaejasme removal in alpine grassland in the Qinghai–Tibetan Plateau. Methods: We investigated the effects of S. chamaejasme removal on ecological stoichiometry by estimating the C:N:P stoichiometry in species, functional group and community levels of the ecosystem. The interactions between different species, functional groups and correlation with soil nutrient, responding to S. chamaejasme removal were also analyzed. Results: For the plants that became dominant after S. chamaejasme removal (SR), N content decreased and their C:N increased. S. chamaejasme removal significantly affected the nutrient stoichiometry of different functional groups. Specifically, Gramineae in the SR sites had decreased N content and N:P, and increased C:N; however, forbs had increased N content, C:P and N:P and decreased P content Submitted 14 November 2019 and C:N. At the community level, N content was lower and C:N higher in SR Accepted 5 May 2020 communities compared to CK. The N content of the plant community was positively 23 June 2020 Published correlated with soil total N content. -

Land Degradation During the Bronze Age in Hexi Corridor (Gansu, China)
Quaternary International 254 (2012) 42e48 Contents lists available at ScienceDirect Quaternary International journal homepage: www.elsevier.com/locate/quaint Land degradation during the Bronze Age in Hexi Corridor (Gansu, China) Zhou Xinying a,b,*, Li Xiaoqiang a,b, John Dodson c, Zhao Keliang a, Pia Atahan c, Sun Nan b,d, Yang Qing b,d a Laboratory of Human Evolution and Archeological Science, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China b State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710075, China c Institute for Environmental Research, The Australian Nuclear Science and Technology Organisation, Sydney, NSW 1001, Australia d Graduate University of Chinese Academy of Sciences, Beijing 100049, China article info abstract Article history: Pollen and charcoal analysis, with high resolution AMS 14C dating, on two sediment sections in the Hexi Available online 23 July 2011 Corridor track the process of settlement development and abandonment during the Bronze Age. The evidence shows that agricultural activity during the Bronze Age caused an increase in farmland and a decrease in the abundance of Artemisia grassland in the Hexi Corridor. Land degradation is probably the main cause for decreased agricultural activity and settlement abandonment. Agriculture- induced soil fertility loss and land salinization contributed to the process of land degradation. However, increasing climate aridity around 4000e3500 cal BP is probably the main initiating cause for the contraction of arable land and vegetation degradation in the Hexi Corridor. Ó 2011 Elsevier Ltd and INQUA. All rights reserved. 1. Introduction Gansu and east Qinghai (Xie, 2002). -

Plants and Natural Products As a Source of Bioactive Molecules
CHAPTER I Introduction Introduction 1. EUPHORBIA L. GENUS 1.1. GENERAL CONSIDERATIONS The Euphorbiaceae family consists of about 300 genera and 7500 species of cosmopolitan distribution, but better developed in tropical and subtropical regions. By far, the largest genus is Euphorbia L. with over 2000 species found in the tropical and subtropical regions of Africa and America, and also in temperate zones worldwide. Euphorbia species (commonly named spurge)1 range from annual or perennial herbs, woody shrubs, trees and succulent plants, characterized by a caustic and toxic milky latex that in contact with the skin may cause inflammation and rash. Some other large genera of this family (in number of species) include Croton (700), Phyllanthus (400), Acalypha (400), Macaranga (250), and Antidesma, Drypetes, Tragia, Jatropha and Manihot, each one with 150 species (Cronquist, 1981; Judd et al, 2002). Euphorbia species have been widely used in traditional medicine all over the world, to treat several diseases, like tumors and warts (Hartwell, 1969). For example, E. peplus and E. platyphylla were used externally to treat dermatosis (Rivera and Óbon, 1995); E. pekinensis 2 is used in traditional Chinese medicine, where it is regarded as one of the 50 fundamental herbs, for the treatment of oedemas and retention of urine (Xue et al, 2007); the roots of E. fisheriana and E. ebracteolata are, along with Stellera chamaejasme (Thymelaeceae), the constituents of a traditional chinese medicine named “Lang-Du”3 that has been used as expectorant and for the treatment of oedema and indigestion (Qin and Xu, 1998). Many members of the Euphorbia genus are of great economic importance as a source of potential petroleum substitutes, due to their rich oil content. -

The 655Th Meeting of the New England Botanical Club, Inc., Being the 882Th Since the Original Organization, Met in the Main Le
New England Botanical Club – Minutes of the 966th Meeting 2 March 2001 Prepared by Don Hudson, Recording Secretary The 739th meeting of the New England Botanical Club, Inc., being the 966th since the original organization, met in the Main Lecture Hall of the Biological Laboratories, Harvard University, and Cambridge, Massachusetts, on Friday, 2 March 2001. President Lisa Standley called the meeting to order at 7:01 PM with 59 members and guests present. After guests were introduced, the chairs of committees were called upon to make their annual reports. Curator of Non-Vascular Plants Nancy Reid reported that the consolidation of the club’s cryptogamic herbarium with that the Farlow is proceeding apace. Fifteen hundred packets were incorporated during the past year alone. Treasurer Harold Brotzman reported that expenses exceeded revenues by over $11,000 during the past year, the balance being made up of earnings from the club’s assets. Graduate Award Chair Pat Swain announced that last year there were two winners of the graduate student award, and that the deadline for the current year had just passed. Library Chair Les Mehrhoff reported on new acquisitions to the club’s library. Curator of Vascular Plants Ray Angelo reported on additions to the club’s herbarium. Les and Arthur Gilman are responsible for the bulk of the additional 202 specimens. The Nominating Committee chaired by David Conant was called upon to present this year’s slate of councilors and officers, and it was adopted by the membership by acclamation. A bumper crop of new club members, including 9 via our business office at Allen Press, was next introduced before President Standley called for announcements.