Platyhelminthes, Monogenea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Pacific Plate Biogeography, with Special Reference to Shorefishes
Pacific Plate Biogeography, with Special Reference to Shorefishes VICTOR G. SPRINGER m SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 367 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoo/ogy Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Taxonomy and Biochemical Genetics of Some African Freshwater Fish Species
_________________________________________________________________________Swansea University E-Theses Taxonomy and biochemical genetics of some African freshwater fish species. Abban, Edward Kofi How to cite: _________________________________________________________________________ Abban, Edward Kofi (1988) Taxonomy and biochemical genetics of some African freshwater fish species.. thesis, Swansea University. http://cronfa.swan.ac.uk/Record/cronfa43062 Use policy: _________________________________________________________________________ This item is brought to you by Swansea University. Any person downloading material is agreeing to abide by the terms of the repository licence: copies of full text items may be used or reproduced in any format or medium, without prior permission for personal research or study, educational or non-commercial purposes only. The copyright for any work remains with the original author unless otherwise specified. The full-text must not be sold in any format or medium without the formal permission of the copyright holder. Permission for multiple reproductions should be obtained from the original author. Authors are personally responsible for adhering to copyright and publisher restrictions when uploading content to the repository. Please link to the metadata record in the Swansea University repository, Cronfa (link given in the citation reference above.) http://www.swansea.ac.uk/library/researchsupport/ris-support/ TAXONOMY AND BIOCHEMICAL GENETICS OF SOME AFRICAN FRESHWATER FISH SPECIES. BY EDWARD KOFI ABBAN A Thesis submitted for the degree of Ph.D. UNIVERSITY OF WALES. 1988 ProQuest Number: 10821454 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. -

Diversity and Length-Weight Relationships of Blenniid Species (Actinopterygii, Blenniidae) from Mediterranean Brackish Waters in Turkey
EISSN 2602-473X AQUATIC SCIENCES AND ENGINEERING Aquat Sci Eng 2019; 34(3): 96-102 • DOI: https://doi.org/10.26650/ASE2019573052 Research Article Diversity and Length-Weight relationships of Blenniid Species (Actinopterygii, Blenniidae) from Mediterranean Brackish Waters in Turkey Deniz İnnal1 Cite this article as: Innal, D. (2019). Diversity and length-weight relationships of Blenniid Species (Actinopterygii, Blenniidae) from Mediterranean Brackish Waters in Turkey. Aquatic Sciences and Engineering, 34(3), 96-102. ABSTRACT This study aims to determine the species composition and range of Mediterranean Blennies (Ac- tinopterygii, Blenniidae) occurring in river estuaries and lagoon systems of the Mediterranean coast of Turkey, and to characterise the length–weight relationship of the specimens. A total of 15 sites were surveyed from November 2014 to June 2017. A total of 210 individuals representing 3 fish species (Rusty blenny-Parablennius sanguinolentus, Freshwater blenny-Salaria fluviatilis and Peacock blenny-Salaria pavo) were sampled from five (Beşgöz Creek Estuary, Manavgat River Es- tuary, Karpuzçay Creek Estuary, Köyceğiz Lagoon Lake and Beymelek Lagoon Lake) of the locali- ties investigated. The high juvenile densities of S. fluviatilis in Karpuzçay Creek Estuary and P. sanguinolentus in Beşgöz Creek Estuary were observed. Various threat factors were observed in five different native habitats of Blenny species. The threats on the habitat and the population of the species include the introduction of exotic species, water ORCID IDs of the authors: pollution, and more importantly, the destruction of habitats. Five non-indigenous species (Prus- D.İ.: 0000-0002-1686-0959 sian carp-Carassius gibelio, Eastern mosquitofish-Gambusia holbrooki, Redbelly tilapia-Copt- 1Burdur Mehmet Akif Ersoy odon zillii, Stone moroko-Pseudorasbora parva and Rainbow trout-Oncorhynchus mykiss) were University, Department of Biology, observed in the sampling sites. -
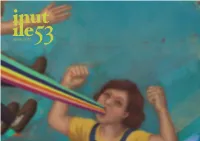
Numero53.Pdf
INUTILE aprile 2013, numero 53 Supplemento al #4205 di PressItalia.net, registrazione presso il Tribunale di Perugia #33 del 5 maggio 2006, pubblicazione trimestrale a cura di INUTILE » ASSOCIAZIONE CULTURALE la redazione leonardo azzolini, marco montanaro, nicolò porcelluzzi, alessandro romeo, matteo scandolin, tamara viola hanno collaborato francesca ballarini, fabio deotto, andrea maggiolo, gianluca nativo, andrea pomella correzioni elisa sottana copertina yuanyuan yang {yuanyuanyang.com} layout leonardo azzolini {leonardoazzolini.com} stampa Le Colibrì - Agenzia di stampa, Gubbio {[email protected]} abbonamenti rivistainutile.it/abbonarsi wild wild web rivistainutile.it twitter.com/inutileonline vimeo.com/inutile Il presente opuscolo è diffuso sotto la disciplina della licenza CREATIVE COMMONS Attribuzione - Non commerciale - Non opere derivate 2.5 Italia. La licenza integrale è disponibile a questo url: http://tinyurl.com/8g7sw5 3 editoriale 4 counselling gianluca nativo 7 via rinaldi 46, ninopoli francesca ballarini 10 quelli che sanno si salvano marco montanaro 16 the mars volta: una storia di confini fabio deotto 24 micronarrativa andrea maggiolo editoriale C’è Gianluca Nativo col suo racconto in grado di sal- la redazione tare da qui a là senza farti mai fermare; c’è Francesca Ballarini con la sua Ninopoli, che c’ha fatto innamo- La copertina di questo numero è un arcobaleno per rare; c’è Marco Montanaro che ci ha fatto riflettere, noi e per voi: per quelli che sono stanchi e abbat- come sempre fa lui; c’è Fabio Deotto che cerca di tuti, la siamo andati a recuperare direttamente da metabolizzare lo scioglimento dei Mars Volta; c’è la Yuanyuan Yang, artista cinese che lavora a New York. -

Edna in a Bottleneck: Obstacles to Fish Metabarcoding Studies in Megadiverse Freshwater 3 Systems 4 5 Authors: 6 Jake M
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.05.425493; this version posted January 7, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Title: 2 eDNA in a bottleneck: obstacles to fish metabarcoding studies in megadiverse freshwater 3 systems 4 5 Authors: 6 Jake M. Jackman1, Chiara Benvenuto1, Ilaria Coscia1, Cintia Oliveira Carvalho2, Jonathan S. 7 Ready2, Jean P. Boubli1, William E. Magnusson3, Allan D. McDevitt1* and Naiara Guimarães 8 Sales1,4* 9 10 Addresses: 11 1Environment and Ecosystem Research Centre, School of Science, Engineering and Environment, 12 University of Salford, Salford, M5 4WT, UK 13 2Centro de Estudos Avançados de Biodiversidade, Instituto de Ciências Biológicas, Universidade 14 Federal do Pará, Belém, Brazil 15 3Coordenação de Biodiversidade, Instituto Nacional de Pesquisas da Amazônia, Manaus, 16 Amazonas, Brazil 17 4CESAM - Centre for Environmental and Marine Studies, Departamento de Biologia Animal, 18 Faculdade de Ciências da Universidade de Lisboa, Lisbon, Portugal 19 20 *Corresponding authors: 21 Naiara Guimarães Sales, [email protected] 22 Allan McDevitt, [email protected] 23 24 Running title: Obstacles to eDNA surveys in megadiverse systems 25 26 Keywords: Amazon, barcoding gap, freshwater, MiFish, Neotropics, reference database, 27 taxonomic resolution 28 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.05.425493; this version posted January 7, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

View/Download
CICHLIFORMES: Cichlidae (part 3) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 6.0 - 30 April 2021 Order CICHLIFORMES (part 3 of 8) Family CICHLIDAE Cichlids (part 3 of 7) Subfamily Pseudocrenilabrinae African Cichlids (Haplochromis through Konia) Haplochromis Hilgendorf 1888 haplo-, simple, proposed as a subgenus of Chromis with unnotched teeth (i.e., flattened and obliquely truncated teeth of H. obliquidens); Chromis, a name dating to Aristotle, possibly derived from chroemo (to neigh), referring to a drum (Sciaenidae) and its ability to make noise, later expanded to embrace cichlids, damselfishes, dottybacks and wrasses (all perch-like fishes once thought to be related), then beginning to be used in the names of African cichlid genera following Chromis (now Oreochromis) mossambicus Peters 1852 Haplochromis acidens Greenwood 1967 acies, sharp edge or point; dens, teeth, referring to its sharp, needle-like teeth Haplochromis adolphifrederici (Boulenger 1914) in honor explorer Adolf Friederich (1873-1969), Duke of Mecklenburg, leader of the Deutsche Zentral-Afrika Expedition (1907-1908), during which type was collected Haplochromis aelocephalus Greenwood 1959 aiolos, shifting, changing, variable; cephalus, head, referring to wide range of variation in head shape Haplochromis aeneocolor Greenwood 1973 aeneus, brazen, referring to “brassy appearance” or coloration of adult males, a possible double entendre (per Erwin Schraml) referring to both “dull bronze” color exhibited by some specimens and to what -

View/Download
CICHLIFORMES: Cichlidae (part 5) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 10.0 - 11 May 2021 Order CICHLIFORMES (part 5 of 8) Family CICHLIDAE Cichlids (part 5 of 7) Subfamily Pseudocrenilabrinae African Cichlids (Palaeoplex through Yssichromis) Palaeoplex Schedel, Kupriyanov, Katongo & Schliewen 2020 palaeoplex, a key concept in geoecodynamics representing the total genomic variation of a given species in a given landscape, the analysis of which theoretically allows for the reconstruction of that species’ history; since the distribution of P. palimpsest is tied to an ancient landscape (upper Congo River drainage, Zambia), the name refers to its potential to elucidate the complex landscape evolution of that region via its palaeoplex Palaeoplex palimpsest Schedel, Kupriyanov, Katongo & Schliewen 2020 named for how its palaeoplex (see genus) is like a palimpsest (a parchment manuscript page, common in medieval times that has been overwritten after layers of old handwritten letters had been scraped off, in which the old letters are often still visible), revealing how changes in its landscape and/or ecological conditions affected gene flow and left genetic signatures by overwriting the genome several times, whereas remnants of more ancient genomic signatures still persist in the background; this has led to contrasting hypotheses regarding this cichlid’s phylogenetic position Pallidochromis Turner 1994 pallidus, pale, referring to pale coloration of all specimens observed at the time; chromis, a name -

Coptodon Zillii (Redbelly Tilapia) Ecological Risk Screening Summary
Redbelly Tilapia (Coptodon zillii) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, May 2019 Revised, September 2019 Web Version, 11/18/2019 Photo: J. Hoover, Waterways Experiment Station, U.S. Army Corp of Engineers. Public domain. Available: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=485. (May 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019a): “Africa and Eurasia: South Morocco, Sahara, Niger-Benue system, rivers Senegal, Sassandra, Bandama, Boubo, Mé, Comoé, Bia, Ogun and Oshun, Volta system, Chad-Shari system [Teugels and Thys van den Audenaerde 1991], middle Congo River basin in the Ubangi, Uele [Thys van den Audenaerde 1964], Itimbiri, Aruwimi [Thys van den Audenaerde 1964; Decru 2015], Lindi- 1 Tshopo [Decru 2015] and Wagenia Falls [Moelants 2015] in Democratic Republic of the Congo, Lakes Albert [Thys van den Audenaerde 1964] and Turkana, Nile system and Jordan system [Teugels and Thys van den Audenaerde 1991].” Froese and Pauly (2019a) list the following countries as part of the native range of Coptodon zillii: Algeria, Benin, Cameroon, Central African Republic, Chad, Democratic Republic of the Congo, Egypt, Ghana, Guinea, Guinea-Bissau, Israel, Ivory Coast, Jordan, Kenya, Lebanon, Liberia, Mali, Mauritania, Morocco, Niger, Nigeria, Senegal, Sierra Leone, Sudan, Togo, Tunisia, Uganda, and Western Sahara. Status in the United States From NatureServe (2019): “Introduced and established in ponds and other waters in Maricopa County, Arizona; irrigation canals in Coachella, Imperial, and Palo Verde valleys, California; and headwater springs of San Antonio River, Bexar County, Texas; common (Page and Burr 1991). Established also in the Carolinas, Hawaii, and possibly in Florida and Nevada (Robins et al. -

View/Download
CICHLIFORMES: Cichlidae (part 2) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 4.0 - 30 April 2021 Order CICHLIFORMES (part 2 of 8) Family CICHLIDAE Cichlids (part 2 of 7) Subfamily Pseudocrenilabrinae African Cichlids (Abactochromis through Greenwoodochromis) Abactochromis Oliver & Arnegard 2010 abactus, driven away, banished or expelled, referring to both the solitary, wandering and apparently non-territorial habits of living individuals, and to the authors’ removal of its one species from Melanochromis, the genus in which it was originally described, where it mistakenly remained for 75 years; chromis, a name dating to Aristotle, possibly derived from chroemo (to neigh), referring to a drum (Sciaenidae) and its ability to make noise, later expanded to embrace cichlids, damselfishes, dottybacks and wrasses (all perch-like fishes once thought to be related), often used in the names of African cichlid genera following Chromis (now Oreochromis) mossambicus Peters 1852 Abactochromis labrosus (Trewavas 1935) thick-lipped, referring to lips produced into pointed lobes Allochromis Greenwood 1980 allos, different or strange, referring to unusual tooth shape and dental pattern, and to its lepidophagous habits; chromis, a name dating to Aristotle, possibly derived from chroemo (to neigh), referring to a drum (Sciaenidae) and its ability to make noise, later expanded to embrace cichlids, damselfishes, dottybacks and wrasses (all perch-like fishes once thought to be related), often used in the names of African cichlid genera following Chromis (now Oreochromis) mossambicus Peters 1852 Allochromis welcommei (Greenwood 1966) in honor of Robin Welcomme, fisheries biologist, East African Freshwater Fisheries Research Organization (Jinja, Uganda), who collected type and supplied ecological and other data Alticorpus Stauffer & McKaye 1988 altus, deep; corpus, body, referring to relatively deep body of all species Alticorpus geoffreyi Snoeks & Walapa 2004 in honor of British carcinologist, ecologist and ichthyologist Geoffrey Fryer (b. -

Montana Kaimin, March 8-14, 2017 Students of the University of Montana, Missoula
University of Montana ScholarWorks at University of Montana Associated Students of the University of Montana Montana Kaimin, 1898-present (ASUM) 3-8-2017 Montana Kaimin, March 8-14, 2017 Students of the University of Montana, Missoula Let us know how access to this document benefits ouy . Follow this and additional works at: https://scholarworks.umt.edu/studentnewspaper Recommended Citation Students of the University of Montana, Missoula, "Montana Kaimin, March 8-14, 2017" (2017). Montana Kaimin, 1898-present. 6937. https://scholarworks.umt.edu/studentnewspaper/6937 This Newspaper is brought to you for free and open access by the Associated Students of the University of Montana (ASUM) at ScholarWorks at University of Montana. It has been accepted for inclusion in Montana Kaimin, 1898-present by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. MARCH 8-14, 2017 2017 8-14, MARCH Lunch gets Hot Pocket Faith in free a second life heirarchy throws page 5 page 10 page 12 | MONTANAKAIMIN.COM | MONTANAKAIMIN.COM THE LAST BEST COLLEGE NEWS COLLEGE BEST THE LAST | @montanakaimin @montanakaimin | facebook.com/montanakaimin Yeezy Season Sneaker culture still running strong Page 8 OPINION The Montana Kaimin is a weekly independent student newspaper at the University of Montana. THIS DAY For comments, corrections or letters to the editor, contact [email protected] or call (406) 243-4310. in History For advertising opportunities, contact Each week the Kaimin will take a look back in [email protected] or call (406) 243-6541. time to see what we were reporting about in the old days.