NIH Public Access Author Manuscript Proteomics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Initial Sequencing and Comparative Analysis of the Mouse Genome
articles Initial sequencing and comparative analysis of the mouse genome Mouse Genome Sequencing Consortium* *A list of authors and their af®liations appears at the end of the paper ........................................................................................................................................................................................................................... The sequence of the mouse genome is a key informational tool for understanding the contents of the human genome and a key experimental tool for biomedical research. Here, we report the results of an international collaboration to produce a high-quality draft sequence of the mouse genome. We also present an initial comparative analysis of the mouse and human genomes, describing some of the insights that can be gleaned from the two sequences. We discuss topics including the analysis of the evolutionary forces shaping the size, structure and sequence of the genomes; the conservation of large-scale synteny across most of the genomes; the much lower extent of sequence orthology covering less than half of the genomes; the proportions of the genomes under selection; the number of protein-coding genes; the expansion of gene families related to reproduction and immunity; the evolution of proteins; and the identi®cation of intraspecies polymorphism. With the complete sequence of the human genome nearly in hand1,2, covering about 90% of the euchromatic human genome, with about the next challenge is to extract the extraordinary trove of infor- 35% in ®nished form1. Since then, progress towards a complete mation encoded within its roughly 3 billion nucleotides. This human sequence has proceeded swiftly, with approximately 98% of information includes the blueprints for all RNAs and proteins, the genome now available in draft form and about 95% in ®nished the regulatory elements that ensure proper expression of all genes, form. -

Associated 16P11.2 Deletion in Drosophila Melanogaster
ARTICLE DOI: 10.1038/s41467-018-04882-6 OPEN Pervasive genetic interactions modulate neurodevelopmental defects of the autism- associated 16p11.2 deletion in Drosophila melanogaster Janani Iyer1, Mayanglambam Dhruba Singh1, Matthew Jensen1,2, Payal Patel 1, Lucilla Pizzo1, Emily Huber1, Haley Koerselman3, Alexis T. Weiner 1, Paola Lepanto4, Komal Vadodaria1, Alexis Kubina1, Qingyu Wang 1,2, Abigail Talbert1, Sneha Yennawar1, Jose Badano 4, J. Robert Manak3,5, Melissa M. Rolls1, Arjun Krishnan6,7 & 1234567890():,; Santhosh Girirajan 1,2,8 As opposed to syndromic CNVs caused by single genes, extensive phenotypic heterogeneity in variably-expressive CNVs complicates disease gene discovery and functional evaluation. Here, we propose a complex interaction model for pathogenicity of the autism-associated 16p11.2 deletion, where CNV genes interact with each other in conserved pathways to modulate expression of the phenotype. Using multiple quantitative methods in Drosophila RNAi lines, we identify a range of neurodevelopmental phenotypes for knockdown of indi- vidual 16p11.2 homologs in different tissues. We test 565 pairwise knockdowns in the developing eye, and identify 24 interactions between pairs of 16p11.2 homologs and 46 interactions between 16p11.2 homologs and neurodevelopmental genes that suppress or enhance cell proliferation phenotypes compared to one-hit knockdowns. These interac- tions within cell proliferation pathways are also enriched in a human brain-specific network, providing translational relevance in humans. Our study indicates a role for pervasive genetic interactions within CNVs towards cellular and developmental phenotypes. 1 Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, PA 16802, USA. 2 Bioinformatics and Genomics Program, The Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA 16802, USA. -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

Neurologic Outcomes in Friedreich Ataxia: Study of a Single-Site Cohort E415
Volume 6, Number 3, June 2020 Neurology.org/NG A peer-reviewed clinical and translational neurology open access journal ARTICLE Neurologic outcomes in Friedreich ataxia: Study of a single-site cohort e415 ARTICLE Prevalence of RFC1-mediated spinocerebellar ataxia in a North American ataxia cohort e440 ARTICLE Mutations in the m-AAA proteases AFG3L2 and SPG7 are causing isolated dominant optic atrophy e428 ARTICLE Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy revisited: Genotype-phenotype correlations of all published cases e434 Academy Officers Neurology® is a registered trademark of the American Academy of Neurology (registration valid in the United States). James C. Stevens, MD, FAAN, President Neurology® Genetics (eISSN 2376-7839) is an open access journal published Orly Avitzur, MD, MBA, FAAN, President Elect online for the American Academy of Neurology, 201 Chicago Avenue, Ann H. Tilton, MD, FAAN, Vice President Minneapolis, MN 55415, by Wolters Kluwer Health, Inc. at 14700 Citicorp Drive, Bldg. 3, Hagerstown, MD 21742. Business offices are located at Two Carlayne E. Jackson, MD, FAAN, Secretary Commerce Square, 2001 Market Street, Philadelphia, PA 19103. Production offices are located at 351 West Camden Street, Baltimore, MD 21201-2436. Janis M. Miyasaki, MD, MEd, FRCPC, FAAN, Treasurer © 2020 American Academy of Neurology. Ralph L. Sacco, MD, MS, FAAN, Past President Neurology® Genetics is an official journal of the American Academy of Neurology. Journal website: Neurology.org/ng, AAN website: AAN.com CEO, American Academy of Neurology Copyright and Permission Information: Please go to the journal website (www.neurology.org/ng) and click the Permissions tab for the relevant Mary E. -

Genome-Wide Transcriptional Sequencing Identifies Novel Mutations in Metabolic Genes in Human Hepatocellular Carcinoma DAOUD M
CANCER GENOMICS & PROTEOMICS 11 : 1-12 (2014) Genome-wide Transcriptional Sequencing Identifies Novel Mutations in Metabolic Genes in Human Hepatocellular Carcinoma DAOUD M. MEERZAMAN 1,2 , CHUNHUA YAN 1, QING-RONG CHEN 1, MICHAEL N. EDMONSON 1, CARL F. SCHAEFER 1, ROBERT J. CLIFFORD 2, BARBARA K. DUNN 3, LI DONG 2, RICHARD P. FINNEY 1, CONSTANCE M. CULTRARO 2, YING HU1, ZHIHUI YANG 2, CU V. NGUYEN 1, JENNY M. KELLEY 2, SHUANG CAI 2, HONGEN ZHANG 2, JINGHUI ZHANG 1,4 , REBECCA WILSON 2, LAUREN MESSMER 2, YOUNG-HWA CHUNG 5, JEONG A. KIM 5, NEUNG HWA PARK 6, MYUNG-SOO LYU 6, IL HAN SONG 7, GEORGE KOMATSOULIS 1 and KENNETH H. BUETOW 1,2 1Center for Bioinformatics and Information Technology, National Cancer Institute, Rockville, MD, U.S.A.; 2Laboratory of Population Genetics, National Cancer Institute, National Cancer Institute, Bethesda, MD, U.S.A.; 3Basic Prevention Science Research Group, Division of Cancer Prevention, National Cancer Institute, Bethesda, MD, U.S.A; 4Department of Biotechnology/Computational Biology, St. Jude Children’s Research Hospital, Memphis, TN, U.S.A.; 5Department of Internal Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea; 6Department of Internal Medicine, University of Ulsan College of Medicine, Ulsan University Hospital, Ulsan, Korea; 7Department of Internal Medicine, College of Medicine, Dankook University, Cheon-An, Korea Abstract . We report on next-generation transcriptome Worldwide, liver cancer is the fifth most common cancer and sequencing results of three human hepatocellular carcinoma the third most common cause of cancer-related mortality (1). tumor/tumor-adjacent pairs. -

IGFBP3 Goat Polyclonal Antibody – TA303275 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA303275 IGFBP3 Goat Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: WB Recommended Dilution: ELISA: 1:64,000. WB: 0.02-0.06µg/ml. Reactivity: Human (Expected from sequence similarity: Mouse, Rat, Dog) Host: Goat Isotype: IgG Clonality: Polyclonal Immunogen: Peptide with sequence C-RYKVDYESQSTDTQN, from the internal region of the protein sequence according to NP_001013416.1; NP_000589.2. Formulation: Supplied at 0.5 mg/ml in Tris saline, 0.02% sodium azide, pH7.3 with 0.5% bovine serum albumin. Purification: Purified from goat serum by ammonium sulphate precipitation followed by antigen affinity chromatography using the immunizing peptide. Supplied at 0.5 mg/ml in Tris saline, 0.02% sodium azide, pH7.3 with 0.5% bovine serum albumin. Aliquot and store at -20°C. Minimize freezing and thawing. Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Gene Name: insulin like growth factor binding protein 3 Database Link: NP_000589 Entrez Gene 16009 MouseEntrez Gene 24484 RatEntrez Gene 100855619 DogEntrez Gene 3486 Human P17936 This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 IGFBP3 Goat Polyclonal Antibody – TA303275 Background: This gene is a member of the insulin-like growth factor binding protein (IGFBP) family and encodes a protein with an IGFBP domain and a thyroglobulin type-I domain. -
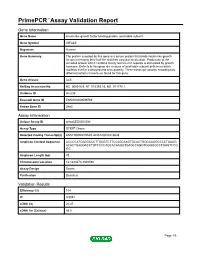
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name insulin-like growth factor binding protein, acid labile subunit Gene Symbol IGFALS Organism Human Gene Summary The protein encoded by this gene is a serum protein that binds insulin-like growth factors increasing their half-life and their vascular localization. Production of the encoded protein which contains twenty leucine-rich repeats is stimulated by growth hormone. Defects in this gene are a cause of acid-labile subunit deficiency which maifests itself in a delayed and slow puberty. Three transcript variants encoding two different isoforms have been found for this gene. Gene Aliases ALS RefSeq Accession No. NC_000016.9, NT_010393.16, NG_011778.1 UniGene ID Hs.839 Ensembl Gene ID ENSG00000099769 Entrez Gene ID 3483 Assay Information Unique Assay ID qHsaCED0003592 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000215539, ENST00000415638 Amplicon Context Sequence ACCCCATCAGGCCCTTGCGTCTTCCAGCAAGTGCACTGGGCAGGCCCCTGAGG ACACTGAGGACCTGTCCCCAGCACAAGGTGAGCCAGGTGGGGGCCTGAGTCCG GG Amplicon Length (bp) 78 Chromosome Location 16:1840473-1840580 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 104 R2 0.9991 cDNA Cq 26.47 cDNA Tm (Celsius) 85.5 Page 1/5 PrimePCR™Assay Validation Report gDNA Cq 24.22 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report IGFALS, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt -

Insulin-Like Growth Factors: Actions on the Skeleton
61 1 Journal of Molecular S Yakar et al. IGFs and bone 61:1 T115–T137 Endocrinology THEMATIC REVIEW 40 YEARS OF IGF1 Insulin-like growth factors: actions on the skeleton Shoshana Yakar1, Haim Werner2 and Clifford J Rosen3 1David B. Kriser Dental Center, Department of Basic Science and Craniofacial Biology, New York University College of Dentistry, New York, New York, USA 2Department of Human Molecular Genetics and Biochemistry, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel 3Maine Medical Center Research Institute, Scarborough, Maine, USA Correspondence should be addressed to S Yakar: [email protected] This paper forms part of a special section on 40 years of IGF1. The guest editors for this section were Derek LeRoith and Emily Gallagher. Abstract The discovery of the growth hormone (GH)-mediated somatic factors (somatomedins), Key Words insulin-like growth factor (IGF)-I and -II, has elicited an enormous interest primarily f insulin-like among endocrinologists who study growth and metabolism. The advancement of f osteoblast molecular endocrinology over the past four decades enables investigators to re-examine f osteoclast and refine the established somatomedin hypothesis. Specifically, gene deletions, f osteocyte transgene overexpression or more recently, cell-specific gene-ablations, have enabled f chondrocyte investigators to study the effects of the Igf1 and Igf2 genes in temporal and spatial manners. The GH/IGF axis, acting in an endocrine and autocrine/paracrine fashion, is the major axis controlling skeletal growth. Studies in rodents have clearly shown that IGFs regulate bone length of the appendicular skeleton evidenced by changes in chondrocytes of the proliferative and hypertrophic zones of the growth plate. -

Constitutional Delay of Growth and Puberty Is Not Commonly Associated
European Journal of Endocrinology (2008) 158 473–477 ISSN 0804-4643 CLINICAL STUDY Constitutional delay of growth and puberty is not commonly associated with mutations in the acid labile subunit gene I Banerjee1, D Hanson2, R Perveen2, A Whatmore3, G C Black2 and P E Clayton1,3 1Department of Paediatric Endocrinology, Royal Manchester Children’s Hospital, Pendlebury, Manchester M27 4HA, UK, 2Academic Unit of Medical Genetics and 3Endocrine Science Research Group, University of Manchester, Manchester M13 9NT, UK (Correspondence should be addressed to I Banerjee; Email: [email protected]) Abstract Objectives: Constitutional delay of growth and puberty (CDGP) is a common clinical condition that may be inherited as an autosomal dominant, recessive or X-linked trait. However, single-gene defects underlying CDGP have not yet been identified. A small number of children (to date 10) with modest growth failure and in the majority delayed puberty, a phenotype similar to that of CDGP, have been reported to carry mutations in the IGF acid labile subunit (IGFALS) gene which encodes the ALS, a part of the ternary complex carrying IGF-I in the circulation. The aim of our study was to screen a well- characterised CDGP cohort exhibiting a range of growth retardation and pubertal delay for pathogenic sequence variants in IGFALS. Design and methods: We used denaturing high performance liquid chromatography (dHPLC) to screen for IGFALS mutations in DNA samples from 90 children (80 males) with CDGP of predominantly White European origin. DNA fragments generating abnormal waveforms were directly sequenced. Results:NoIGFALS mutation was identified in the coding sequences or exon–intron boundaries in our CDGP cohort. -

Identification of Novel Target Genes by an Epigenetic Reactivation Screen of Renal Cancer Inmaculada Ibanez De Caceres,1 Essel Dulaimi,1 Amanda M
Research Article Identification of Novel Target Genes by an Epigenetic Reactivation Screen of Renal Cancer Inmaculada Ibanez de Caceres,1 Essel Dulaimi,1 Amanda M. Hoffman,1 Tahseen Al-Saleem,2 RobertG. Uzzo, 1 and Paul Cairns1,2 Departments of 1Surgical Oncology and 2Pathology, Fox Chase Cancer Center, Philadelphia, Pennsylvania Abstract common mechanism for the silencing of tumor suppressor gene Aberrant promoter hypermethylation is a common mecha- (TSG) in cancer cells (5, 6). Several TSG have been identified as hypermethylated with associated loss of expression in renal cancer nism for inactivation of tumor suppressor genes in cancer INK4a by a candidate approach. The VHL and p16 TSG are inactivated cells. To generate a global profile of genes silenced by hypermethylation in renal cell cancer (RCC), we did an by promoter hypermethylation in up to 20% of clear cell (7) and 10% expression microarray-based analysis of genes reactivated in of all RCC (8), respectively. However, to date, few genes have been found to be frequently hypermethylated in RCC. The RASSF1A the 786-0, ACHN, HRC51, and HRC59 RCC lines after treatment Timp-3 with the demethylating drug 5-aza-2 deoxycytidine and gene is hypermethylated in 27% to 56% (9–11), and the gene histone deacetylation inhibiting drug trichostatin A. Between is hypermethylated in 58% to 78%, of primary RCC (11, 12). By 111 to 170 genes were found to have at least 3-fold up- definition, a candidate gene approach has resulted in the exam- regulation of expression after treatment in each cell line. To ination of a limited number of genes for epigenetic alteration (11). -

A New Structural Model of the Acid-Labile Subunit: Pathogenetic Mechanisms of Short Stature-Causing Mutations
213 A new structural model of the acid-labile subunit: pathogenetic mechanisms of short stature-causing mutations Alessia David*, Lawrence A Kelley* and Michael J E Sternberg Centre for Integrative Systems Biology and Bioinformatics, Division of Molecular Biosciences, Department of Life Sciences, Imperial College London, London SW7 2AZ, UK (Correspondence should be addressed to A David; Email: [email protected]) *(A David and L A Kelley equally contributed to this work) Abstract The acid-labile subunit (ALS) is the main regulator of IGF1 and IGF2 bioavailability. ALS deficiency caused by mutations in the ALS (IGFALS) gene often results in mild short stature in adulthood. Little is known about the ALS structure–function relationship. A structural model built in 1999 suggested a doughnut shape, which has never been observed in the leucine- rich repeat (LRR) superfamily, to which ALS belongs. In this study, we built a new ALS structural model, analysed its glycosylation and charge distribution and studied mechanisms by which missense mutations affect protein structure. We used three structure prediction servers and integrated their results with information derived from ALS experimental studies. The ALS model was built at high confidence using Toll-like receptor protein templates and resembled a horseshoe with an extensively negatively charged concave surface. Enrichment in prolines and disulphide bonds was found at the ALS N- and C-termini. Moreover, seven N-glycosylation sites were identified and mapped. ALS mutations were predicted to affect protein structure by causing loss of hydrophobic interactions (p.Leu134Gln), alteration of the amino acid backbone (p.Leu241Pro, p.Leu172Phe and p.Leu244Phe), loss of disulphide bridges (p.Cys60Ser and p.Cys540Arg), change in structural constrains (p.Pro73Leu), creation of novel glycosylation sites (p.Asp440Asn) or alteration of LRRs (p.Asn276Ser). -

Analysis of Acid-Labile Subunit and Its Usefulness in Pediatrics
Review Arch Argent Pediatr 2017;115(4):391-398 / 391 Analysis of acid-labile subunit and its usefulness in pediatrics Verónica E. Zaidman, Biochemista ABSTRACT in serum bound to a high molecular The acid-labile subunit (ALS) is an 85 kDa glycoprotein that belongs to the leucine-rich weight (150 kDa) ternary complex. In repeat superfamily. It mainly circulates in such complex, ALS is associated with si se traduce sacar serum bound to a high molecular weight ternary some of the proteins of the insulin- Abstract complex. like growth factor (IGF) family and The main and most widely studied function of ALS is to prolong the half-life of the binary one of the insulin-like growth factor- complex formed by insulin-like growth factors binding proteins (IGFBP): IGFBP3 or type 1 and 2 and its transport proteins 3 and 5. IGFBP5.2,3 ALS serum levels are lower in neonates, reach a The IGF family is comprised of peak in late puberty, and then slowly decrease throughout adulthood. ALS deficiency has 2 peptide hormones synthesized by consequences on growth, hydrocarbon and bone the liver: IGF1 and IGF2. These share metabolism, and, in some cases, it affects pubertal approximately 50% of their amino development. To date, 25 patients with complete acid sequence and a large portion of ALS deficiency due to IGFALS gene mutations have been found. their sequence displays homology to Key words: acid-labile subunit, insulin-like growth proinsulin but, unlike the latter, they factor-1, short stature. retain C-peptide and are therefore http://dx.doi.org/10.5546/aap.2017.eng.391 longer.