Case Report - Disulfoton Poisoning of Beef Cattle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hawaii on Two Foliage Plants, Dwarf Brassaia Diazinon Plant After Spots
in Japan and England will be given by Tosh Fu- {Brassaia arboricola) and Dwarf Ti {Cordyline chikami of O.M. Scotts and Ray McMicken of B. terminalis 'Madameandre') to determine their Hayman Co., respectively. phototoxicity to selected insecticides and acara- cides. Plants, growing in 6-inch pots, were treated Farwest Show by submerging the aerial portions of the plant in Farwest Nursery, Garden, and Supply Show water suspensions of 7 pesticides for 15seconds. will be September 8-10, 1975, at the Memorial Granular formulations of 2 pesticides were ap Coliseum in Portland, Oregon. For information plied to the soil surface. Materials, at 2X stand contact: Farwest Nursery Show, Suite GA-7, ard rates, were as follows: 222 S.W. Harrison, Protland, OR. 97201. Amount formulation per: ASHS The 72d annual meeting of ASHS (American Material and formulation 1-gallon water 6-inch pot Society for Horticultural Science) will be held in chlorobenzilate 4E 2t — Honolulu, September 8 to 13, 1975, at the dicofol (Kelthane) 35WP 2T - Sheraton-Waikiki Hotel. Meeting concurrently Pentac 50WP 2T — with ASHS will be the American Horticulture carbaryl (Sevin) 50WP 2T - Society. The University of Hawaii will host the diazinon AG500 (48% EC) 2t - meeting with Dr. Richard Bullock, general chair dimethoate (Cygon) 2E 2t — man. Dr. Henry Nakasone will serve as assistant Volck Oil Supreme 2T - general chairman, Dr. Phil Parvin as local arrange aldicarb (Temik) 10G - 1.5t ments chairman, and Dr. Richard Criley as pro disulfoton (Di-Syston) 15G - 1.5t gram chairman. Neighbor island tours will be untreated controls - conducted following the meetings. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

US Environmental Protection Agency Office of Pesticide Programs
US Environmental Protection Agency Office of Pesticide Programs Reregistration Eligibility Decision for Disulfoton When EPA concluded the organophosphate (OP) cumulative risk assessment in July 2006, all tolerance reassessment and reregistration eligibility decisions for individual OP pesticides were considered complete. OP Interim Reregistration Eligibility Decisions (IREDs), therefore, are considered completed REDs. OP tolerance reassessment decisions (TREDs) also are considered completed. Combined PDF document consists of the following: • Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides (July 31, 2006) • Disulfoton IRED UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES MEMORANDUM DATE: July 31, 2006 SUBJECT: Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides FROM: Debra Edwards, Director Special Review and Reregistration Division Office of Pesticide Programs TO: Jim Jones, Director Office of Pesticide Programs As you know, EPA has completed its assessment of the cumulative risks -

Disulfoton CAS #: 298-04-4
Review Date: 10/20/2013 disulfoton CAS #: 298-04-4 Type Organophosphate insecticide. Controls Selective insecticide especially effective on sucking insects - aphids, beetles, billbugs, borers, grasshoppers, leafhoppers, leafminers, mealybugs, midge, mites, moths, psyllids, scale, thrips, webworm, wireworm, and whiteflies. Mode of Action Systemic insecticide that is absorbed through plant roots and is an acetylcholinesterase (AChE) inhibitor in insects. Thurston County Review Summary: The insecticide active ingredient disulfotton is rated high in hazard and products containing it fail Thurston County's pesticide review criteria. Disulfoton is rated high in hazard for its mutagenic potential. Risk to humans and wildlife from the use of disulfoton insecticides is rated moderate for the only remaining EPA registered product. According to the EPA's January 2010 registration review decision document for disulfoton, the registrants of all pesticide products containing disulfoton were voluntarily cancelled. No new products will be manufactured but existing products can be sold (Reference 3). As of the date of this review, the only existing product containing disulfoton registered for use in the states of Washington and Oregon has the EPA registration number 72155-49, which is a granular product for use on residential ornamentals including rose bushes, shrubs, and flowerbeds. MOBILITY Property Value Reference Value Rating Water Solubility (mg/L) 25 4 Moderate - low Soil Sorption (Kd=mL/g) Value not found Organic Sorption (Koc=mL/g) 383 to 888 1 Moderate - high Mobility Summary: Disulfoton is not very soluble in water and is expected to adhere moderately to organic soil. The hazard for disulfoton to move off the site of application with rain or irrigation water is rated moderate if the product is left on the surface soil, and low if it is incorporated into the soil and watered in. -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

List of Class 1 Designated Chemical Substances
List of Class 1 Designated Chemical Substances *1:CAS numbers are to be solely as references. They may be insufficient or lacking, in case there are multiple chemical substances. No. Specific Class 1 CAS No. (PRTR Chemical (*1) Name Law) Substances 1 - zinc compounds(water-soluble) 2 79-06-1 acrylamide 3 140-88-5 ethyl acrylate 4 - acrylic acid and its water-soluble salts 5 2439-35-2 2-(dimethylamino)ethyl acrylate 6 818-61-1 2-hydroxyethyl acrylate 7 141-32-2 n-butyl acrylate 8 96-33-3 methyl acrylate 9 107-13-1 acrylonitrile 10 107-02-8 acrolein 11 26628-22-8 sodium azide 12 75-07-0 acetaldehyde 13 75-05-8 acetonitrile 14 75-86-5 acetone cyanohydrin 15 83-32-9 acenaphthene 16 78-67-1 2,2'-azobisisobutyronitrile 17 90-04-0 o-anisidine 18 62-53-3 aniline 19 82-45-1 1-amino-9,10-anthraquinone 20 141-43-5 2-aminoethanol 21 1698-60-8 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one; chloridazon 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano- 22 120068-37-3 4[(trifluoromethyl)sulfinyl]pyrazole; fipronil 23 123-30-8 p-aminophenol 24 591-27-5 m-aminophenol 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one; 25 21087-64-9 metribuzin 26 107-11-9 3-amino-1-propene 27 41394-05-2 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one; metamitron 28 107-18-6 allyl alcohol 29 106-92-3 1-allyloxy-2,3-epoxypropane 30 - n-alkylbenzenesulfonic acid and its salts(alkyl C=10-14) 31 - antimony and its compounds 32 120-12-7 anthracene 33 1332-21-4 asbestos ○ 34 4098-71-9 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate 35 78-84-2 isobutyraldehyde -

1 of 3 GC+LC-USA
Updated: 07/18/2016 1 of 3 GC+LC-USA Limit of Quantitation (LOQ): 0.010 mg/kg (ppm) Sample Types: Low Fat Content Samples Minimum Sample Size: 100 grams (~1/4 pound). Certain products require more for better sample representation. Instrument: GC-MS/MS and LC-MS/MS Turnaround: 24-48 hours Accreditation: Part of AGQ USA's ISO/IEC 17025 Accreditation Scope 4,4'-Dichlorobenzophenone Bupirimate Cyantraniliprole Diflufenican Abamectin Buprofezin Cyazofamid Dimethoate Acephate Butachlor Cycloate Dimethoate (Sum) Acequinocyl Butocarboxim Cycloxydim Dimethomorph Acetamiprid Butralin Cyflufenamid Diniconazole Acetochlor Cadusafos Cyfluthrin Dinocap Acrinathrin Captafol Cymoxanil Dinotefuran Alachlor Captan Cyproconazole Diphenylamine Aldicarb Captan (Sum) Cyprodinil Disulfoton Aldicarb (Sum) Carbaryl Cyromazine Disulfoton (Sum) Aldicarb-sulfone Carbofuran DDD-o,p Disulfoton-sulfone Aldicarb-sulfoxide Carbofuran-3-hydroxy DDD-p,p +DDT-o,p Disulfoton-sulfoxide Aldrin Carbophenothion DDE-o,p Ditalimfos Ametryn Carbosulfan DDE-p,p Diuron Amitraz Carboxine DDT (Sum) Dodemorph Atrazine Carfentrazone-ethyl DDT-p,p Dodine Azadirachtin Chinomethionat DEET Emamectin Benzoate Azamethiphos Chlorantraniliprole Deltamethrin Endosulfan (A+B+Sulf) Azinphos-ethyl Chlordane Demeton Endosulfan Alfa Azinphos-methyl Chlordane Trans Demeton-S-methyl-sulfone Endosulfan Beta Azoxystrobin Chlorfenapyr Desmedipham Endosulfan Sulfate Benalaxyl Chlorfenson Diafenturion Endrin Ben-Carb-TPM (Sum) Chlorfenvinphos Dialifos EPN Bendiocarb Chlorfluazuron Diazinon Epoxiconazole -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -

Method 614: the Determination of Organophosphorus Pesticides in Municipal and Industrial Wastewater
www.epa.gov 1992 Method 614: The Determination of Organophosphorus Pesticides in Municipal and Industrial Wastewater Method 614 The Determination of Organophosphorus Pesticides in Municipal and Industrial Wastewater Method 614 The Determination of Organophosphorus Pesticides in Municipal and Industrial Wastewater 1. SCOPE AND APPLICATION 1.1 This method covers the determination of certain organophosphorus pesticides. The following parameters can be determined by this method: Parameter STORET No. CAS No. Azinphos methyl 39580 86-50-0 Demeton 39560 8065-48-3 Diazinon 39570 333-41-5 Disulfoton 39010 298-04-4 Ethion - 563-12-2 Malathion 39530 121-75-5 Parathion ethyl 39540 56-38-2 Parathion methyl 39600 298-00-0 1.2 This is a gas chromatographic (GC) method applicable to the determination of the compounds listed above in industrial and municipal discharges as provided under 40 CFR 136.1. Any modification of this method beyond those expressly permitted shall be considered a major modification subject to application and approval of alternative test procedures under 40 CFR 136.4 and 136.5. 1.3 The method detection limit (MDL, defined in Section 15) for several parameters are listed in Table 1. The MDL for a specific wastewater may differ from those listed, depending upon the nature of interferences in the sample matrix. 1.4 The sample extraction and concentration steps in this method are essentially the same as in Method 617. Thus, a single sample may be extracted to measure the parameters included in the scope of both of these methods. When cleanup is required, the concentration levels must be high enough to permit selecting aliquots, as necessary, in order to apply appropriate cleanup procedures. -

Environmental Protection Agency Pt. 355, App. A
Environmental Protection Agency Pt. 355, App. A Release means any spilling, leaking, the facility is located. In the absence pumping, pouring, emitting, emptying, of a SERC for a State or Indian Tribe, discharging, injecting, escaping, leach- the Governor or the chief executive of- ing, dumping, or disposing into the en- ficer of the tribe, respectively, shall be vironment (including the abandonment the SERC. Where there is a cooperative or discarding of barrels, containers, agreement between a State and a and other closed receptacles) of any Tribe, the SERC shall be the entity hazardous chemical, EHS, or CERCLA identified in the agreement. hazardous substance. Solution means any aqueous or or- Reportable quantity means, for any ganic solutions, slurries, viscous solu- CERCLA hazardous substance, the tions, suspensions, emulsions, or quantity established in Table 302.4 of 40 pastes. CFR 302.4, for such substance. For any State means any State of the United EHS, reportable quantity means the States, the District of Columbia, the quantity established in Appendices A Commonwealth of Puerto Rico, Guam, and B of this part for such substance. American Samoa, the United States Unless and until superseded by regula- Virgin Islands, the Northern Mariana tions establishing a reportable quan- Islands, any other territory or posses- tity for newly listed EHSs or CERCLA sion over which the United States has hazardous substances, a weight of 1 jurisdiction and Indian Country. pound shall be the reportable quantity. Threshold planning quantity means, SERC means the State Emergency for a substance listed in Appendices A Response Commission for the State in and B of this part, the quantity listed which the facility is located except in the column ‘‘threshold planning where the facility is located in Indian quantity’’ for that substance. -
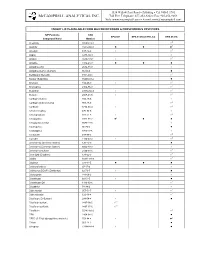
Compounds/Target Lists Detail
1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC. Toll Free Telephone: 877-252-9262 • Fax: 925-252-9269 Web: www.mccampbell.com • E-mail: [email protected] TARGET LISTS AVAILABLE FROM MAI FOR NITROGEN & PHOSPHOROUS PESTICIDES N/P Pesticide CAS EPA 507 EPA 8141A (CTR List) EPA 8141A Compound Name Number Acephate 30560-19-1 zZ Alachlor 15972-60-8 z z zZ Ametryn 834-12-8 z zZ Aspon 3244-90-4 z Atraton 1610-17-9 z zZ Atrazine 1912-24-9 z z z Azinphos ethyl 2642-71-9 z Azinphos methyl (Guthion) 86-50-0 z Benfluralin (Benefin) 1861-40-1 zZ Bolstar (Sulprofos) 35400-43-2 z Bromacil 314-40-9 z zZ Bromophos 2104-96-3 zZ Butachlor 23184-66-9 z zZ Butylate 2008-41-5 z zZ Carbophenothion 786-19-6 z Carbophenothion methyl 953-17-3 zZ Carboxin 5234-68-4 z zZ Chlorfenvinphos 470-90-6 z Chloropropham 101-21-3 z zZ Chlorpyrifos 2921-88-2 zZ z z Chlorpyrifos methyl 5598-13-0 z Coumaphos 56-72-4 z Crotoxyphos 7700-17-6 z Crufomate 299-86-5 zZ Cycloate 1134-23-2 z zZ Demeton-O (Demeton isomer) 126-75-0 z Demeton-S (Demeton isomer) 8065-48-3 z Demeton-S sulfone 2496-91-5 zZ Deet (Off) (Dephere) 134-62-3 zZ Dialifor 10311-84-9 zZ Diazinon 333-41-5 z z z Dichlorofenthion 97-17-6 z Dichlorvos (DDVP) (Dichlorfos) 62-73-7 z z Dicrotophos 141-66-2 z Dimethoate 60-51-5 z z Dimethoate OA 1113-02-6 zZ Dioxathion 78-34-2 z Diphenamid 957-51-7 z zZ Diphenylamine 122-39-4 zZ Disulfoton (Di-Syston) 298-04-4 z z Disulfoton sulfone 2497-06-5 zX1 Disulfoton sulfoxide 2497-07-6 zX1 Ethalflurin 55283-68-6 zZ EPN 2104-64-5 z EPTC (S-Ethyl dipropylthiocarbamate) 759-94-4 z zZ Ethion 563-12-2 z Ethoprop 13194-48-4 z z 1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC.