Drug Pipeline Monthly Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Postmortem Distribution of Vardenafil (Levitra | in an Aviation
Journal of Analytical Toxicology, Vol. 31, July/August 2007 The Postmortem Distribution of Vardenafil (Levitra| in an Aviation Accident Victim with an Unusually High Blood Concentration* Robert D. Johnson ~, Russell J. Lewis, and Mike K. Angler Downloaded from https://academic.oup.com/jat/article/31/6/328/682815 by guest on 27 September 2021 Civil Aerospace Medical Institute, Federal Aviation Administration, Analytical Toxicology and Accident Research Laboratory, AAM-610, CAMI Building, 6500 S. MacArthur Blvd., Oklahoma City, Oklahoma 73169-6901 I Abstract phodiesterase type 5 enzyme (PDE5) found predominantly in the penile corpus cavernosum (2-7). Vardenafil (tevitra) is one of the most widely prescribed Vardenafil undergoes hepatic metabolism, producing the treatments for erectile dysfunction. This report presents a active desethyl metabolite M1. M1 contributes to the ob- rapid and reliable method for the identification and quantification served pharmacological effects provided by vardenafil, as M1 of vardenafil in postmortem fluids and tissues, applies this method exhibits approximately 30% of the potency of the parent to a postmortem case, and describes the distribution of vardenafil in various fluids and tissues.This procedure utilizes sildenafil-d8, drug (1). Under steady-state conditions, the plasma concen- which is structurally closely related to vardenafil, as an tration of M1 is approximately 26% of that seen for vardenafil internal standard for more accurate and reliable quantitation. (1). After oral administration of vardenafil, peak plasma con- The method incorporates solid-phaseextraction and liquid centrations are obtained within 30-60 min (1). Vardenafil chromatography-tandem mass spectrometry (MS) and and its active metabolite have a terminal half-life of approx- MS-MS-MS utilizing an atmospheric pressure chemical imately 4-5 h (1). -

Infectious Diseases
2013 MEDICINES IN DEVELOPMENT REPORT Infectious Diseases A Report on Diseases Caused by Bacteria, Viruses, Fungi and Parasites PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Research Evolves Against Infectious Diseases with Nearly 400 Medicines and Vaccines in Testing Throughout history, infectious diseases hepatitis C that inhibits the enzyme have taken a devastating toll on the lives essential for viral replication. and well-being of people around the • An anti-malarial drug that has shown Medicines in Development world. Caused when pathogens such activity against Plasmodium falci- For Infectious Diseases as bacteria or viruses enter a body and parum malaria which is resistant to multiply, infectious diseases were the current treatments. Application leading cause of death in the United Submitted States until the 1920s. Today, vaccines • A potential new antibiotic to treat methicillin-resistant Staphylococcus Phase III and infectious disease treatments have proven to be effective treatments in aureus (MRSA). Phase II many cases, but infectious diseases still • A novel treatment that works by Phase I pose a very serious threat to patients. blocking the ability of the smallpox Recently, some infectious pathogens, virus to spread to other cells, thus 226 such as pseudomonas bacteria, have preventing it from causing disease. become resistant to available treatments. Infectious diseases may never be fully Diseases once considered conquered, eradicated. However, new knowledge, such as tuberculosis, have reemerged new technologies, and the continuing as a growing health threat. commitment of America’s biopharma- America’s biopharmaceutical research ceutical research companies can help companies are developing 394 medicines meet the continuing—and ever-changing and vaccines to combat the many threats —threat from infectious diseases. -

Inclusion and Exclusion Criteria for Each Key Question
Supplemental Table 1: Inclusion and exclusion criteria for each key question Chronic HBV infection in adults ≥ 18 year old (detectable HBsAg in serum for >6 months) Definition of disease Q1 Q2 Q3 Q4 Q5 Q6 Q7 HBV HBV infection with infection and persistent compensated Immunoactive Immunotolerant Seroconverted HBeAg HBV mono-infected viral load cirrhosis with Population chronic HBV chronic HBV from HBeAg to negative population under low level infection infection anti-HBe entecavir or viremia tenofovir (<2000 treatment IU/ml) Adding 2nd Stopped antiviral therapy antiviral drug Interventions and Entecavir compared Antiviral Antiviral therapy compared to continued compared to comparisons to tenofovir therapy therapy continued monotherapy Q1-2: Clinical outcomes: Cirrhosis, decompensated liver disease, HCC and death Intermediate outcomes (if evidence on clinical outcomes is limited or unavailable): HBsAg loss, HBeAg seroconversion and Outcomes HBeAg loss Q3-4: Cirrhosis, decompensated liver disease, HCC, relapse (viral and clinical) and HBsAg loss Q5: Renal function, hypophosphatemia and bone density Q6: Resistance, flare/decompensation and HBeAg loss Q7: Clinical outcomes: Cirrhosis, decompensated liver disease, HCC and death Study design RCT and controlled observational studies Acute HBV infection, children and pregnant women, HIV (+), HCV (+) or HDV (+) persons or other special populations Exclusions such as hemodialysis, transplant, and treatment failure populations. Co treatment with steroids and uncontrolled studies. Supplemental Table 2: Detailed Search Strategy: Ovid Database(s): Embase 1988 to 2014 Week 37, Ovid MEDLINE(R) In-Process & Other Non- Indexed Citations and Ovid MEDLINE(R) 1946 to Present, EBM Reviews - Cochrane Central Register of Controlled Trials August 2014, EBM Reviews - Cochrane Database of Systematic Reviews 2005 to July 2014 Search Strategy: # Searches Results 1 exp Hepatitis B/dt 26410 ("hepatitis B" or "serum hepatitis" or "hippie hepatitis" or "injection hepatitis" or 2 178548 "hepatitis type B").mp. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -
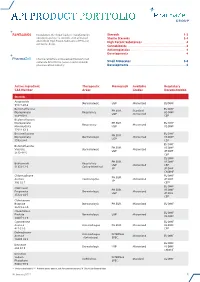
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC. -

Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR)
Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR) October 20, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004-2020 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Manufacturer Indication(s) Corticosteroids – Ophthalmic Topical dexamethasone (Maxidex®)1 Alcon/Novartis . -

Keeping up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A
Keeping Up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A. Shlom, PharmD, BCPS Senior Vice President & Director Clinical Pharmacy Program | Acurity, Inc. Privileged and Confidential April 10, 2019 Privileged and Confidential Program Objectives By the end of the presentation, the pharmacist or pharmacy technician participant will be able to: ▪ Identify orphan drugs and first-in-class medications approved by the FDA in 2018. ▪ List five new drugs and their indications. ▪ Identify the place in therapy for three novel monoclonal antibodies. ▪ Discuss at least two new medications that address public health concerns. Dr. Shlom does not have any conflicts of interest in regard to this presentation. Both trade names and generic names will be discussed throughout the presentation Privileged and Confidential 2018 NDA Approvals (NMEs/BLAs) ▪ Lutathera (lutetium Lu 177 dotatate) ▪ Braftovi (encorafenib) ▪ Vizimpro (dacomitinib) ▪ Biktarvy (bictegravir, emtricitabine, ▪ TPOXX (tecovirimat) ▪ Libtayo (cemiplimab-rwic) tenofovir, ▪ Tibsovo (ivosidenib) ▪ Seysara (sarecycline) alafenamide) ▪ Krintafel (tafenoquine) ▪ Nuzyra (omadacycline) ▪ Symdeko (tezacaftor, ivacaftor) ▪ Orilissa (elagolix sodium) ▪ Revcovi (elapegademase-lvir) ▪ Erleada (apalutamide) ▪ Omegaven (fish oil triglycerides) ▪ Tegsedi (inotersen) ▪ Trogarzo (ibalizumab-uiyk) ▪ Mulpleta (lusutrombopag) ▪ Talzenna (talazoparib) ▪ Ilumya (tildrakizumab-asmn) ▪ Poteligeo (mogamulizumab-kpkc) ▪ Xofluza (baloxavir marboxil) ▪ Tavalisse (fostamatinib disodium) ▪ Onpattro (patisiran) -

Joel L. Young, M.D
Joel L. Young, M.D. 441 South Livernois Road, Suite 100 Rochester Hills, Michigan 48307 Phone: 248-608-8800 / Fax: 248-608-2490 / E-mail: [email protected] Professional History 2000 – Present: Chief Medical Officer and Founder, Clinical Trials Group at the Rochester Center for Behavioral Medicine, Rochester Hills, MI 1993 – Present: Medical Director and Founder, Rochester Center for Behavioral Medicine, Rochester Hills, MI (www.rcbm.net). 2008 (Current): Clinical Associate Professor of Psychiatry, Wayne State University, Detroit, MI. 2000 – 2007: Medical Director, Crittenton Network for Behavioral Health, Rochester, MI. 2000 – 2002: Chief of Staff, Department of Psychiatry, Crittenton Hospital, Rochester, MI. July, 1993 – 1997: Medical Director, Psychiatric Emergency Services, Crittenton Hospital. July, 1992 – June, 1993: Chief Resident of Adult Services, Department of Psychiatry, University of Michigan Hospitals, Ann Arbor, MI. Oct. 1991-Sept. 1993: Unit Psychiatrist, Bon Secours Adolescent Mental Health Unit, Grosse Pointe, MI. August, 1991 – 1996: Consulting Psychiatrist, Beacon Hill Clinic, Birmingham, MI. July, 1991 – June, 1992: Consulting Psychiatrist, Washtenaw County Community Mental Health Services, Ann Arbor, MI. July, 1990 – June, 1992: House Officer, Department of Psychiatry, University of Michigan Hospitals. June, 1989 – June, 1990: Intern, Departments of Internal Medicine, Pediatrics and Psychiatry, University of Michigan Hospitals. Boards 2018 Fellow: American Board of Psychiatry and Neurology 2017 Re-certification of Geriatric Qualifications by the American Board of Psychiatry and Neurology through 2027. 2017 Re-certification of Forensic Qualifications by the American Board of Psychiatry and Neurology through 2027 2014 Re-Certification by the American Board of Psychiatry and Neurology 2007 Re-certification by the American Board of Adolescent Psychiatry. -

DCAT MEMBER COMPANY MEETING LOCATOR V. 4
DCAT MEMBER COMPANY MEETING LOCATOR v. 4 THE BENJAMIN Sancilio Pharmaceuticals Company, Inc. Sri Krishna Pharmaceuticals Ltd. Amino Chemicals Ltd. Zydus Pharmaceuticals (USA) Inc. Apogee Pharma, Inc. C2 PHARMA INTERCONTINENTAL BARCLAY Calyx Chemicals & Pharmaceuticals Ltd. AbbVie* ChemCon GmbH ACIC Pharmaceuticals Inc. Concord Biotech Limited Advitech SA Dipharma Francis Srl Amneal Pharmaceuticals LLC DSM Sinochem Pharmaceuticals ALP Pharm F.I.S. - Fabbrica Italiana Sintetici S.p.A. AMRI Jost Chemical Co. Asymchem Inc. Jubilant Pharma Capsugel, Now a Lonza Company* PharmSource, A GlobalData Company CBC AMERICAS Corp. PolyPeptide Group CellMark USA, LLC ROHNER Inc. Charioteer Pharmaceutical Co., Ltd., Zhejiang FIFTY NYC, A AFFINA HOTEL Chemical and Pharmaceutical Solutions Chiral Quest Corp. Chartwell Pharmaceuticals, LLC Croda, Inc. HOTEL 48LEX DFE Pharma AB BioTechnologies, Inc. DPL-US AiPing Pharmaceutical, Inc. EQ Esteve Almac Evonik Corporation Aptuit LLC FAREVA SA AZAD Fine Chemicals Ltd. Flavine North America, Inc. Cambridge Isotope Laboratories, Inc. Formosa Laboratories, Inc. CMC Biologics Grifols International S.A. Groupe Parima Hainan Poly Pharm. Co., Ltd. Navin Fluorine International Limited Harris Pharmaceutical Qualicaps, Inc. Helm AG RC2 Pharma Connect LLC Hetero USA, Inc. Recipharm Hikal, Ltd. Recro Gainesville LLC Interchem Corporation Reed-Lane, Inc. Inventia Healthcare PVT LTD Please note: Some DCAT member companies have requested not to be listed in the locator. (*) indicates member companies with Business Meeting Spaces in more than one hotel. INTERCONTINENTAL BARCLAY CONT'D PiSA BioPharm, Inc. SPI Pharma Inc. Johnson Matthey Tapemark Kingchem Life Science LLC Unither Pharmaceutical Legacy Pharmaceutical Packaging Uquifa S.A. Lonza AG* Neuland Laboratories Ltd. LOTTE NY PALACE Orion Group AbbVie* Par Pharmaceutical, Inc. -

Supported by an Educational Grant from Sunovion Pharmaceuticals Inc. Faculty
Supported by an educational grant from Sunovion Pharmaceuticals Inc. Faculty Leslie Citrome, MD, MPH C. Brendan Montano, MD Clinical Professor of Psychiatry and CT Clinical Research Behavioral Sciences Director, Principal Investigator New York Medical College Private Practice, Internal Medicine Valhalla, New York Cromwell, Connecticut Faculty Disclosure • Dr. Citrome: Consultant—Acadia, Alkermes, Allergan, Intra-Cellular Therapeutics, Janssen, Lundbeck, Merck, Neurocrine, Noven, Osmotica, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva, Vanda; Royalties—Springer Healthcare (book), UpToDate (reviewer), Wiley (Editor in Chief, International Journal of Clinical Practice); Shareholder (and spouse)—Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer; Speaker—Acadia, Alkermes, Allergan, Janssen, Lundbeck, Merck, Neurocrine, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva. • Dr. Montano: Consultant—Allergan, Shire/Takeda Pharmaceutical Company Ltd., Sunovion Pharmaceuticals Inc., Arbor Pharmaceuticals Ltd.; Research Support—Allergan, Avanir, Sunovion Pharmaceuticals Inc., Tonix, BioHaven, Axsome Therapeutics, Arbor Pharmaceuticals Ltd.; Speakers Bureau— Allergan, Shire/Takeda Pharmaceutical Company Ltd., Arbor Pharmaceutical Ltd. Disclosure • The faculty have been informed of their responsibility to disclose to the audience if they will be discussing off-label or investigational use(s) of drugs, products, and/or devices (any use not approved by the US Food and Drug Administration). – The off-label and investigational use of antidepressants, topiramate,