Outcome Measures in Stroke Rehabilitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Brief March 2017 Publication #2017-16
Research Brief March 2017 Publication #2017-16 Flourishing From the Start: What Is It and How Can It Be Measured? Kristin Anderson Moore, PhD, Child Trends Christina D. Bethell, PhD, The Child and Adolescent Health Measurement Introduction Initiative, Johns Hopkins Bloomberg School of Every parent wants their child to flourish, and every community wants its Public Health children to thrive. It is not sufficient for children to avoid negative outcomes. Rather, from their earliest years, we should foster positive outcomes for David Murphey, PhD, children. Substantial evidence indicates that early investments to foster positive child development can reap large and lasting gains.1 But in order to Child Trends implement and sustain policies and programs that help children flourish, we need to accurately define, measure, and then monitor, “flourishing.”a Miranda Carver Martin, BA, Child Trends By comparing the available child development research literature with the data currently being collected by health researchers and other practitioners, Martha Beltz, BA, we have identified important gaps in our definition of flourishing.2 In formerly of Child Trends particular, the field lacks a set of brief, robust, and culturally sensitive measures of “thriving” constructs critical for young children.3 This is also true for measures of the promotive and protective factors that contribute to thriving. Even when measures do exist, there are serious concerns regarding their validity and utility. We instead recommend these high-priority measures of flourishing -

The Origin of the Peculiarities of the Vietnamese Alphabet André-Georges Haudricourt
The origin of the peculiarities of the Vietnamese alphabet André-Georges Haudricourt To cite this version: André-Georges Haudricourt. The origin of the peculiarities of the Vietnamese alphabet. Mon-Khmer Studies, 2010, 39, pp.89-104. halshs-00918824v2 HAL Id: halshs-00918824 https://halshs.archives-ouvertes.fr/halshs-00918824v2 Submitted on 17 Dec 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Published in Mon-Khmer Studies 39. 89–104 (2010). The origin of the peculiarities of the Vietnamese alphabet by André-Georges Haudricourt Translated by Alexis Michaud, LACITO-CNRS, France Originally published as: L’origine des particularités de l’alphabet vietnamien, Dân Việt Nam 3:61-68, 1949. Translator’s foreword André-Georges Haudricourt’s contribution to Southeast Asian studies is internationally acknowledged, witness the Haudricourt Festschrift (Suriya, Thomas and Suwilai 1985). However, many of Haudricourt’s works are not yet available to the English-reading public. A volume of the most important papers by André-Georges Haudricourt, translated by an international team of specialists, is currently in preparation. Its aim is to share with the English- speaking academic community Haudricourt’s seminal publications, many of which address issues in Southeast Asian languages, linguistics and social anthropology. -

Dutchess County Public Transit Route L
Route L: Main Street Shuttle Please see map on page 44 / Favor ver el mapa en la página 44 LUNES–SABADO 9 NORTH / NORTE MONDAY–SATURDAY / Ave Violet Ave EASTBOUND: Poughkeepsie to Stop & Shop or Adams Fairacre Farms / HACIA EL ESTE: Poughkeepsie a Stop & Shop or Adams Fairacre Farms Poughkeepsie Train Station DCPT Routes: A,B,C,D,E,H,J,K,L,P,PRL Map not to scale Amtrak, MTA Metro-North Mapa no a escala Dutchess County Transit Hub Interfaith DCPT Routes: A,B,C,D,E,H,J,K,L,M,P,PRL Adams Fairacre Farms & Route 44 (Arrives) POUGHKEEPSIE Washington St & Mansion St (Interfaith Towers) POUGHKEEPSIE Poughkeepsie StationTrain POUGHKEEPSIE Main St & Worrall Ave POUGHKEEPSIE Colledgeview Ave & Fairmont Ave (Vassar College) POUGHKEEPSIE Route 44 & Burnett Blvd POUGHKEEPSIE Stop Shop & (Arrives) POUGHKEEPSIE Towers County Dutchess Hub Transit (Departs) POUGHKEEPSIE Stop # 1 2 3 4 5 6 7 8 Mill St 1 286 3 306 308 131 313 311 2 SEE DOWNTOWN POUGHKEEPSIE INSET ON PAGE 12 Market 6:45 6:48 6:52 7:03 7:08 7:11 — 7:15 7:15 7:18 7:22 7:33 7:38 7:41 7:44 — 3 Main St 7:45 7:48 7:52 8:03 8:08 8:11 — 8:15 1 Adams 8:15 8:18 8:22 8:33 8:38 8:41 8:44 — Fairacre 8:45 8:48 8:52 9:03 9:08 9:11 — 9:15 Ea Stop & st- Farms AM 9:15 9:18 9:22 9:33 9:38 9:41 9:44 — We s Shop 9:45 9:48 9:52 10:03 10:08 10:11 — 10:15 Mid-Hudson t A rter Bridge ial 8 10:15 10:18 10:22 10:33 10:38 10:41 10:44 — Main St Arlington 10:45 10:48 10:52 11:03 11:08 11:11 — 11:15 7 11:15 11:18 11:22 11:33 11:38 11:41 11:44 — Innis Ave 11:45 11:48 11:52 12:03 12:08 12:11 — 12:15 D E a s t- W 44 12:15 12:18 -

How Can We Create Environments Where Hate Cannot Flourish?
How can we create environments where hate cannot flourish? Saturday, February 1 9:30 a.m. – 10:00 a.m. Check-In and Registration Location: Field Museum West Entrance 10:00 a.m. – 10:30 a.m. Introductions and Program Kick-Off JoAnna Wasserman, USHMM Education Initiatives Manager Location: Lecture Hall 1 10:30 a.m. – 11:30 a.m. Watch “The Path to Nazi Genocide” and Reflections JoAnna Wasserman, USHMM Education Initiatives Manager Location: Lecture Hall 1 11:30 a.m. – 11:45 a.m. Break and walk to State of Deception 11:45 a.m. – 12:30 p.m. Visit State of Deception Interpretation by Holocaust Survivor Volunteers from the Illinois Holocaust Museum Location: Upper level 12:30 p.m. – 1:00 p.m. Breakout Session: Reflections on Exhibit Tim Kaiser, USHMM Director, Education Initiatives David Klevan, USHMM Digital Learning Strategist JoAnna Wasserman, USHMM Education Initiatives Manager Location: Lecture Hall 1, Classrooms A and B Saturday, February 2 (continued) 1:00 p.m. – 1:45 p.m. Lunch 1:45 p.m. – 2:45 p.m. A Survivor’s Personal Story Bob Behr, USHMM Survivor Volunteer Interviewed by: Ann Weber, USHMM Program Coordinator Location: Lecture Hall 1 2:45 p.m. – 3:00 p.m. Break 3:00 p.m. – 3:45 p.m. Student Panel: Beyond Indifference Location: Lecture Hall 1 Moderator: Emma Pettit, Sustained Dialogue Campus Network Student/Alumni Panelists: Jazzy Johnson, Northwestern University Mary Giardina, The Ohio State University Nory Kaplan-Kelly, University of Chicago 3:45 p.m. – 4:30 p.m. Breakout Session: Sharing Personal Reflections Tim Kaiser, USHMM Director, Education Initiatives David Klevan, USHMM Digital Learning Strategist JoAnna Wasserman, USHMM Education Initiatives Manager Location: Lecture Hall 1, Classrooms A and B 4:30 p.m. -

The Utility of Genetic Risk Scores in Predicting the Onset of Stroke March 2021 6
DOT/FAA/AM-21/24 Office of Aerospace Medicine Washington, DC 20591 The Utility of Genetic Risk Scores in Predicting the Onset of Stroke Diana Judith Monroy Rios, M.D1 and Scott J. Nicholson, Ph.D.2 1. KR 30 # 45-03 University Campus, Building 471, 5th Floor, Office 510 Bogotá D.C. Colombia 2. FAA Civil Aerospace Medical Institute, 6500 S. MacArthur Blvd Rm. 354, Oklahoma City, OK 73125 March 2021 NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents thereof. _________________ This publication and all Office of Aerospace Medicine technical reports are available in full-text from the Civil Aerospace Medical Institute’s publications Web site: (www.faa.gov/go/oamtechreports) Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-21/24 4. Title and Subtitle 5. Report Date March 2021 The Utility of Genetic Risk Scores in Predicting the Onset of Stroke 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Diana Judith Monroy Rios M.D1, and Scott J. Nicholson, Ph.D.2 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) 1 KR 30 # 45-03 University Campus, Building 471, 5th Floor, Office 510, Bogotá D.C. Colombia 11. Contract or Grant No. 2 FAA Civil Aerospace Medical Institute, 6500 S. MacArthur Blvd Rm. 354, Oklahoma City, OK 73125 12. Sponsoring Agency name and Address 13. Type of Report and Period Covered Office of Aerospace Medicine Federal Aviation Administration 800 Independence Ave., S.W. -
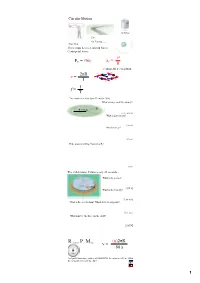
FC = Mac V2 R V = 2Πr T F = T 1
Circular Motion Gravitron Cars Car Turning (has a curse) Rope Skip There must be a net inward force: Centripetal Force v2 F = maC a = C C R Centripetal acceleration 2πR v v v = R v T v 1 f = T The motorcycle rider does 13 laps in 150 s. What is his period? Frequency? R = 30 m [11.5 s, 0.087 Hz] What is his velocity? [16.3 m/s] What is his aC? [8.9 m/s2] If the mass is 180 kg, what is his FC? [1600 N] The child rotates 5 times every 22 seconds... What is the period? 60 kg [4.4 s] What is the velocity? [2.86 m/s] What is the acceleration? Which way does it point? [4.1 m/s2] What must be the force on the child? [245 N] Revolutions Per Minute (n)2πR v = 60 s An Ipod's hard drive rotates at 4200 RPM. Its radius is 0.03 m. What is the velocity in m/s of its edge? 1 Airplane Turning v = 232 m/s (520 mph) aC = 7 g's What is the turn radius? [785 m = 2757 ft] 2 FC Solving Circular Motion Problems 1. Determine FC (FBD) 2 2. Set FC = m v R 3. Solve for unknowns The book's mass is 2 kg it is spun so that the tension is 45 N. What is the velocity of the book in m/s? In RPM? R = 0.75 m [4.11 m/s] [52 RPM] What is the period (T) of the book? [1.15 s] The car is traveling at 25 m/s and the turn's radius is 63 m. -

ISO Basic Latin Alphabet
ISO basic Latin alphabet The ISO basic Latin alphabet is a Latin-script alphabet and consists of two sets of 26 letters, codified in[1] various national and international standards and used widely in international communication. The two sets contain the following 26 letters each:[1][2] ISO basic Latin alphabet Uppercase Latin A B C D E F G H I J K L M N O P Q R S T U V W X Y Z alphabet Lowercase Latin a b c d e f g h i j k l m n o p q r s t u v w x y z alphabet Contents History Terminology Name for Unicode block that contains all letters Names for the two subsets Names for the letters Timeline for encoding standards Timeline for widely used computer codes supporting the alphabet Representation Usage Alphabets containing the same set of letters Column numbering See also References History By the 1960s it became apparent to thecomputer and telecommunications industries in the First World that a non-proprietary method of encoding characters was needed. The International Organization for Standardization (ISO) encapsulated the Latin script in their (ISO/IEC 646) 7-bit character-encoding standard. To achieve widespread acceptance, this encapsulation was based on popular usage. The standard was based on the already published American Standard Code for Information Interchange, better known as ASCII, which included in the character set the 26 × 2 letters of the English alphabet. Later standards issued by the ISO, for example ISO/IEC 8859 (8-bit character encoding) and ISO/IEC 10646 (Unicode Latin), have continued to define the 26 × 2 letters of the English alphabet as the basic Latin script with extensions to handle other letters in other languages.[1] Terminology Name for Unicode block that contains all letters The Unicode block that contains the alphabet is called "C0 Controls and Basic Latin". -

National Rappel Operations Guide
National Rappel Operations Guide 2019 NATIONAL RAPPEL OPERATIONS GUIDE USDA FOREST SERVICE National Rappel Operations Guide i Page Intentionally Left Blank National Rappel Operations Guide ii Table of Contents Table of Contents ..........................................................................................................................ii USDA Forest Service - National Rappel Operations Guide Approval .............................................. iv USDA Forest Service - National Rappel Operations Guide Overview ............................................... vi USDA Forest Service Helicopter Rappel Mission Statement ........................................................ viii NROG Revision Summary ............................................................................................................... x Introduction ...................................................................................................... 1—1 Administration .................................................................................................. 2—1 Rappel Position Standards ................................................................................. 2—6 Rappel and Cargo Letdown Equipment .............................................................. 4—1 Rappel and Cargo Letdown Operations .............................................................. 5—1 Rappel and Cargo Operations Emergency Procedures ........................................ 6—1 Documentation ................................................................................................ -

M.'S Story Jay and Tava Musial, Who Were Living in Michigan at the Time
M.’s Story Jay and Tava Musial, who were living in Michigan at the time, had planned a family trip for Memorial Day weekend, but not before stopping by their daughter’s pediatrician’s office. Tava had noticed her daughter M. Rhapsody frequently had to go to the bathroom. Assuming she had a urinary tract infection, they hoped to get antibiotics and be on their way. M.’s lab work revealed no signs of bacteria but indicated her blood sugar was extremely high. Five days after her eighth birthday, M. was diagnosed with Type 1 diabetes. The Musials’ weekend getaway quickly rerouted to St. John’s Hospital in Detroit where M. spent a full week learning how to manage diabetes. Shortly after M’s diagnosis, the Musial family relocated to Sheppard Air Force Base in Wichita Falls, Texas. Relocating also meant finding a new endocrinologist for M. It was at that first appointment that Dr. John Caras recommended Diabetes Education at United Regional. Diabetes is a disease that affects the whole family, especially when a child is diagnosed. Diabetes Education also provides a support group for kids and their families. “M. comes to every appointment with a smile on her face and has never let her diabetes hold her back on anything,” said Nicole Benz, Diabetes Nurse Educator at United Regional. Children’s Miracle Network at United Regional provides scholarships for kids with Type 1 Diabetes to go to Camp Sweeney. This three-week camp’s goal is that every child who attends camp will not only learn about his or her condition but will find the inner strength each day to do what it takes to live a healthy lifestyle. -

Speech Sound Programme for 'K' / 'C' at the Start of Words
Speech Sound Programme for ‘k’ / ‘c’ at the start of words This programme is for children who are producing a ‘t’ sound in place of a ‘k’ / ‘c’ sound at the start of words. For example, ‘cat’ is produced ‘tat’ and ‘cup’ is produced ‘tup’. ‘k’ is the target sound. ‘t’ is the produced sound. Below are a set of stages to work through with the child. Start with Stage 1 and only move up to the next stage when you are confident that the child has achieved the current stage. It is recommended that the programme is carried out for 15 minutes three times a week. If you would like any advice or you feel that the child is not making progress, please contact the Speech and Language Therapy team on 0151 514 2334. Stage 1 - Listening for the target sound 1. See ‘Picture Set 1’ for sound cue pictures that represent the target sound ‘k’ and the produced sound ‘t’. 2. Place the two pictures (‘k’ and ‘t’) in front of the child. 3. Teach the child the sounds, not the letter names, e.g., say ‘k’ and not ‘k-uh’. 4. Say the two sounds at random and ask the child to point or place a counter on which sound they hear. 5. Repeat this activity a number of times until you are sure the child can consistently hear the difference between the two sounds. Stage 2 - Sorting pictures by their first sound. 1. Now that the child can hear the difference between the two sounds (‘k’ and ‘t’) on their own, try the same with words. -

Impact of Blinding on Estimated Treatment Effects in Randomised Clinical Trials
RESEARCH Impact of blinding on estimated treatment effects in randomised BMJ: first published as 10.1136/bmj.l6802 on 21 January 2020. Downloaded from clinical trials: meta-epidemiological study Helene Moustgaard,1-4 Gemma L Clayton,5 Hayley E Jones,5 Isabelle Boutron,6 Lars Jørgensen,4 David R T Laursen,1-4 Mette F Olsen,4 Asger Paludan-Müller,4 Philippe Ravaud,6 5,7 5,7,8 5,7 1-3 Jelena Savović, , Jonathan A C Sterne, Julian P T Higgins, Asbjørn Hróbjartsson For numbered affiliations see ABSTRACT 1 indicated exaggerated effect estimates in trials end of the article. OBJECTIVES without blinding. Correspondence to: To study the impact of blinding on estimated RESULTS H Moustgaard treatment effects, and their variation between [email protected] The study included 142 meta-analyses (1153 trials). (or @HeleneMoustgaa1 on Twitter trials; differentiating between blinding of patients, The ROR for lack of blinding of patients was 0.91 ORCID 0000-0002-7057-5251) healthcare providers, and observers; detection bias (95% credible interval 0.61 to 1.34) in 18 meta- Additional material is published and performance bias; and types of outcome (the analyses with patient reported outcomes, and 0.98 online only. To view please visit MetaBLIND study). the journal online. (0.69 to 1.39) in 14 meta-analyses with outcomes C ite this as: BMJ 2020;368:l6802 DESIGN reported by blinded observers. The ROR for lack of http://dx.doi.org/10.1136/bmj.l6802 Meta-epidemiological study. blinding of healthcare providers was 1.01 (0.84 to Accepted: 19 November 2019 DATA SOURCE 1.19) in 29 meta-analyses with healthcare provider Cochrane Database of Systematic Reviews (2013-14). -

Threshold Digraphs
Volume 119 (2014) http://dx.doi.org/10.6028/jres.119.007 Journal of Research of the National Institute of Standards and Technology Threshold Digraphs Brian Cloteaux1, M. Drew LaMar2, Elizabeth Moseman1, and James Shook1 1National Institute of Standards and Technology, Gaithersburg, MD 20899 2The College of William and Mary, Williamsburg, VA 23185 [email protected] [email protected] [email protected] [email protected] A digraph whose degree sequence has a unique vertex labeled realization is called threshold. In this paper we present several characterizations of threshold digraphs and their degree sequences, and show these characterizations to be equivalent. Using this result, we obtain a new, short proof of the Fulkerson-Chen theorem on degree sequences of general digraphs. Key words: digraph realizations; Fulkerson-Chen theorem; threshold digraphs. Accepted: May 6, 2014 Published: May 20, 2014 http://dx.doi.org/10.6028/jres.119.007 1. Introduction Creating models of real-world networks, such as social and biological interactions, is a central task for understanding and measuring the behavior of these networks. A usual first step in this type of model creation is to construct a digraph with a given degree sequence. We examine the extreme case of digraph construction where for a given degree sequence there is exactly one digraph that can be created. What follows is a brief introduction to the notation used in the paper. For notation not otherwise defined, see Diestel [1]. We let G=( VE , ) be a digraph where E is a set of ordered pairs called arcs. If (,vw )∈ E, then we say w is an out-neighbor of v and v is an in-neighbor of w.