JILL M DANIEL, Daniel Ph.D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenotyping, Etiological Factors, and Biomarkers: Toward Precision Medicine in Autism Spectrum Disorders David Q
Special Article Phenotyping, Etiological Factors, and Biomarkers: Toward Precision Medicine in Autism Spectrum Disorders David Q. Beversdorf, MD; MISSOURI AUTISM SUMMIT CONSORTIUM* ABSTRACT: Despite the progress made in understanding the biology of autism spectrum disorder (ASD), effective biological interventions for the core symptoms remain elusive. Because of the etiological hetero- geneity of ASD, identification of a “one-size-fits-all” treatment approach will likely continue to be chal- lenging. A meeting was convened at the University of Missouri and the Thompson Center to discuss strategies for stratifying patients with ASD for the purpose of moving toward precision medicine. The “white paper” presented here articulates the challenges involved and provides suggestions for future solutions. (J Dev Behav Pediatr 37:659–673, 2016) Index terms: autism, biomarkers, precision medicine. Significant progress has been made in understanding Phase II clinical trials.5 This perceived “failure” is likely the biology of autism spectrum disorder (ASD) over the due to the etiological heterogeneity of the subjects with past decade. However, effective biological interventions ASD who received the specific treatment. A review of the for the core symptoms remain elusive. Instead of a single data for the arbaclofen study suggests a strong positive or even a small set of causes, a consensus has emerged response for at least a subset of fragile X and patients with that genetic and environmental causes of ASD are likely ASD. Positive responses in some individuals, but other- multifactorial. The genetic architecture of ASD has be- wise statistically nonsignificant beneficial group effects, come increasingly clear and increasingly complex with are characteristic of most of these early pharmacological estimates of at least 1000 genetic alterations associated treatment trials of ASD. -

Autism Diagnosis Differentiates Neurophysiological Responses To
Tye et al. Journal of Neurodevelopmental Disorders (2015) 7:33 DOI 10.1186/s11689-015-9129-2 RESEARCH Open Access Autism diagnosis differentiates neurophysiological responses to faces in adults with tuberous sclerosis complex Charlotte Tye1,2*, Teresa Farroni3,4, Ágnes Volein4, Evelyne Mercure5, Leslie Tucker4, Mark H. Johnson4 and Patrick F. Bolton1,2 Abstract Background: Autism spectrum disorder (ASD) is a common and highly heritable neurodevelopmental disorder that is likely to be the outcome of complex aetiological mechanisms. One strategy to provide insight is to study ASD within tuberous sclerosis complex (TSC), a rare disorder with a high incidence of ASD, but for which the genetic cause is determined. Individuals with ASD consistently demonstrate face processing impairments, but these have not been examined in adults with TSC using event-related potentials (ERPs) that are able to capture distinct temporal stages of processing. Methods: For adults with TSC (n = 14), 6 of which had a diagnosis of ASD, and control adults (n = 13) passively viewed upright and inverted human faces with direct or averted gaze, with concurrent EEG recording. Amplitude and latency of the P1 and N170 ERPs were measured. Results: Individuals with TSC + ASD exhibited longer N170 latencies to faces compared to typical adults. Typical adults and adults with TSC-only exhibited longer N170 latency to inverted versus upright faces, whereas individuals with TSC + ASD did not show latency differences according to face orientation. In addition, individuals with TSC + ASD showed increased N170 latency to averted compared to direct gaze, which was not demonstrated in typical adults. A reduced lateralization was shown for the TSC + ASD groups on P1 and N170 amplitude. -

Sleep As a Translationally-Relevant Endpoint in Studies of Autism Spectrum Disorder (ASD)
www.nature.com/npp NEUROPSYCHOPHARMACOLOGY REVIEWS Sleep as a translationally-relevant endpoint in studies of autism spectrum disorder (ASD) Galen Missig 1, Christopher J. McDougle2,3 and William A. Carlezon Jr.1 Sleep has numerous advantages for aligning clinical and preclinical (basic neuroscience) studies of neuropsychiatric illness. Sleep has high translational relevance, because the same endpoints can be studied in humans and laboratory animals. In addition, sleep experiments are conducive to continuous data collection over long periods (hours/days/weeks) and can be based on highly objective neurophysiological measures. Here, we provide a translationally-oriented review on what is currently known about sleep in the context of autism spectrum disorder (ASD), including ASD-related conditions, thought to have genetic, environmental, or mixed etiologies. In humans, ASD is frequently associated with comorbid medical conditions including sleep disorders. Animal models used in the study of ASD frequently recapitulate dysregulation of sleep and biological (diurnal, circadian) rhythms, suggesting common pathophysiologies across species. As our understanding of the neurobiology of ASD and sleep each become more refined, it is conceivable that sleep-derived metrics may offer more powerful biomarkers of altered neurophysiology in ASD than the behavioral tests currently used in humans or lab animals. As such, the study of sleep in animal models for ASD may enable fundamentally new insights on the condition and represent a basis for strategies that enable the development of more effective therapeutics. Neuropsychopharmacology (2020) 45:90–103; https://doi.org/10.1038/s41386-019-0409-5 INTRODUCTION dysregulation are frequently comorbid and thus also represent A recent estimate suggests that as many as 2.5% of children have important aspects of these conditions [10]. -
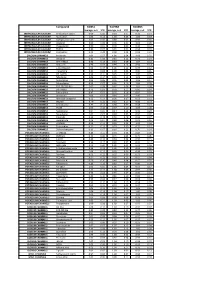
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

Contribution of Multiple Inherited Variants to Autism Spectrum Disorder (ASD) in a Family with 3 Affected Siblings
G C A T T A C G G C A T genes Article Contribution of Multiple Inherited Variants to Autism Spectrum Disorder (ASD) in a Family with 3 Affected Siblings Jasleen Dhaliwal 1 , Ying Qiao 1,2, Kristina Calli 1,2, Sally Martell 1,2, Simone Race 3,4,5, Chieko Chijiwa 1,5, Armansa Glodjo 3,5, Steven Jones 1,6 , Evica Rajcan-Separovic 2,7, Stephen W. Scherer 4 and Suzanne Lewis 1,2,5,* 1 Department of Medical Genetics, University of British Columbia (UBC), Vancouver, BC V6H 3N1, Canada; [email protected] (J.D.); [email protected] (Y.Q.); [email protected] (K.C.); [email protected] (S.M.); [email protected] (C.C.); [email protected] (S.J.) 2 BC Children’s Hospital, Vancouver, BC V5Z 4H4, Canada; [email protected] 3 Department of Pediatrics, University of British Columbia (UBC), Vancouver, BC V6T 1Z7, Canada; [email protected] (S.R.); [email protected] (A.G.) 4 The Centre for Applied Genomics and McLaughlin Centre, Hospital for Sick Children and University of Toronto, Toronto, ON M5G 0A4, Canada; [email protected] 5 BC Children’s and Women’s Health Center, Vancouver, BC V6H 3N1, Canada 6 Michael Smith Genome Sciences Centre, Vancouver, BC V5Z 4S6, Canada 7 Department of Pathology and Laboratory Medicine, University of British Columbia (UBC), Vancouver, BC V6T 1Z7, Canada * Correspondence: [email protected] Abstract: Autism Spectrum Disorder (ASD) is the most common neurodevelopmental disorder in Citation: Dhaliwal, J.; Qiao, Y.; Calli, children and shows high heritability. -

Heritability of Autism Spectrum Disorder in a UK Population-Based Twin Sample
Research Original Investigation Heritability of Autism Spectrum Disorder in a UK Population-Based Twin Sample Emma Colvert, PhD; Beata Tick, MSc; Fiona McEwen, PhD; Catherine Stewart, PhD; Sarah R. Curran, MD; Emma Woodhouse, BSc; Nicola Gillan, BSc; Victoria Hallett, PhD; Stephanie Lietz, PhD; Tracy Garnett, DClinPsych; Angelica Ronald, PhD; Robert Plomin, PhD; Frühling Rijsdijk, PhD; Francesca Happé, PhD; Patrick Bolton, MD Supplemental content at IMPORTANCE Most evidence to date highlights the importance of genetic influences on the jamapsychiatry.com liability to autism and related traits. However, most of these findings are derived from clinically ascertained samples, possibly missing individuals with subtler manifestations, and obtained estimates may not be representative of the population. OBJECTIVES To establish the relative contributions of genetic and environmental factors in liability to autism spectrum disorder (ASD) and a broader autism phenotype in a large population-based twin sample and to ascertain the genetic/environmental relationship between dimensional trait measures and categorical diagnostic constructs of ASD. DESIGN, SETTING, AND PARTICIPANTS We used data from the population-based cohort Twins Early Development Study, which included all twin pairs born in England and Wales from January 1, 1994, through December 31, 1996. We performed joint continuous-ordinal liability threshold model fitting using the full information maximum likelihood method to estimate genetic and environmental parameters of covariance. Twin pairs underwent the following assessments: the Childhood Autism Spectrum Test (CAST) (6423 pairs; mean age, 7.9 years), the Development and Well-being Assessment (DAWBA) (359 pairs; mean age, 10.3 years), the Autism Diagnostic Observation Schedule (ADOS) (203 pairs; mean age, 13.2 years), the Autism Diagnostic Interview–Revised (ADI-R) (205 pairs; mean age, 13.2 years), and a best-estimate diagnosis (207 pairs). -

Autism Diagnosis As Social Process
Drawing a line in the sand: Autism diagnosis as social process Submitted by Jennie Hayes to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Medical Studies December 2019 Submitted by Jennie Hayes, this thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. Signature……………………………………………………………………………… 1 2 This PhD project was funded by a Wellcome Trust Investigator Award ‘Exploring Diagnosis’ 3 4 Abstract This PhD explored how clinicians make diagnostic decisions about autism in secondary care. Symptoms of autism are considered to be widely heterogeneous, meaning that decisions about where the diagnostic threshold lies can be challenging. Diagnosis in the UK is usually undertaken by multi- disciplinary teams (MDTs) and can involve numerous stages of decision-making in different contexts across an extended time period. The process of diagnosis is complex and multi-faceted, and can be particularly challenging when cases are considered ‘borderline’ or where there are coexisting conditions. A qualitative approach was used in four studies. A narrative review of twenty- one clinical guidelines was conducted (study one); observation of eighteen assessment team meetings on four sites was undertaken (studies two and three); and sixteen interviews were conducted with clinicians engaged in autism diagnosis (study four). -

Diagnostic Change and the Genetic Makeup of the Autism Population1
Looping Genomes: Diagnostic Change and the Genetic Makeup of the Autism Population1 Daniel Navon University of California, San Diego Gil Eyal Columbia University This article builds on Hacking’s framework of “dynamic nominal- ism” to show how knowledge about biological etiology can interact with the “kinds of people” delineated by diagnostic categories in ways that “loop” or modify both over time. The authors use historical ma- terials to show how “geneticization” played a crucial role in binding together autism as a biosocial community and how evidence from ge- netics research later made an important contribution to the diagnostic expansion of autism. In the second part of the article, the authors draw on quantitative and qualitative analyses of autism rates over time in several rare conditions that are delineated strictly according to geno- mic mutations in order to demonstrate that these changes in diagnos- tic practice helped to both increase autism’s prevalence and create its enormous genetic heterogeneity. Thus, a looping process that began with geneticization and involved the social effects of genetics research itself transformed the autism population and its genetic makeup. It is by now well recognized that most traits and disease categories do not line up in a straightforward way with characteristics of the human genome ðLock 2005; Wade 2009Þ. Nevertheless, the project of explaining, tracing, 1 This study was conducted with the generous support of the USA-Israel Binational Sci- ence Foundation ðgrant 2010175Þ and a seed grant from the Institute for Social and Economic Research and Policy at Columbia University. We would like to thank the AJS reviewers as well as Peter Bearman, Alberto Cambrosio, James Evans, Uri Shwed, Claire Edington, Daniel Wojtkiewicz, Ayelet Evrony, and audiences at Northwestern © 2016 by The University of Chicago. -

Big Conductance Calcium-Activated Potassium Channel Openers Control Spasticity Without Sedation
British Journal of British Journal of Pharmacology (2017) 174 2662–2681 2662 BJP Pharmacology RESEARCH PAPER Big conductance calcium-activated potassium channel openers control spasticity without sedation Correspondence Professor David Baker, Neuroimmunology Unit, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, 4 Newark Street, Whitechapel, London E1 4AT, UK. E-mail: [email protected] Received 5 January 2017; Revised 27 April 2017; Accepted 17 May 2017 David Baker1,2,* ,GarethPryce1,2,*, Cristina Visintin2,3,SofiaSisay1, Alexander I Bondarenko4,5, WSVanessaHo6, Samuel J Jackson1, Thomas E Williams1, Sarah Al-Izki1, Ioanna Sevastou3, Masahiro Okuyama3, Wolfgang F Graier4, Lesley A Stevenson6,CarolynTanner7,RuthRoss7, Roger G Pertwee7, Christopher M Henstridge8,AndrewJIrving8, Jesse Schulman9,KeithPowell9,MarkDBaker1, Gavin Giovannoni1,2 and David L Selwood3,* 1Neuroimmunology Unit, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK, 2Department of Neuroinflammation, UCL Institute of Neurology, University College London, London, UK, 3Department of Medicinal Chemistry, UCL Wolfson Institute for Biomedical Research, University College London, London, UK, 4Institute of Molecular Biology and Biochemistry, Medical University of Graz, Graz, Austria, 5A.A. Bogomoletz Institute of Physiology, Kiev, Ukraine, 6Vascular Biology Research Centre. St. George’s, University of London, London, UK, 7Department of Biomedical Sciences, -

Functional Analysis of Penicillium Paxilli Genes Required for Biosynthesis of Paxilline
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Functional analysis of Penicillium paxilli genes required for biosynthesis of paxilline This thesis is presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) In Biochemistry at Massey University, Palmerston North, New Zealand Sanjay Saikia 2006 ABSTRACT Paxilline belongs to a large, structurally and functionally diverse group of indole-diterpenes and is synthesised by the filamentous fungus Penicillium paxilli. A gene cluster for paxilline biosynthesis in P. paxilli has been identified and characterised. However, none of the steps proposed in the biosynthesis of paxilline or paxilline-like indole-diterpenes have been validated . In some diterpene-producing fi lamentous fungi, including P. paxilli, two distinct copies of geranylgeranyl diphosphate (GGPP) synthase, that catalyses the committed step in diterpene biosynthesis, have been identified . However, the biological significance of the presence of two distinct GGPP synthases is not known. In this study, biochemical analysis of the paxilline gene products in P. paxilli and subcellular localisation of the two P. paxilli GGPP synthases, Ggs1 and PaxG, were carried out. Transfer of constructs containing different combinations of pax genes into a pax cluster negative deletion derivative of P. paxilli identified four Pax proteins that are required for the biosynthesis of a paxilline intermediate, paspaline. These proteins are PaxG, a GGPP synthase, PaxM, a FAD-dependent monooxygenase, PaxB, a putative membrane protein, and PaxC, a prenyltransferase. -

The Heritability of Autism Spectrum Disorder R01CA203809 from the National Cancer Institute (NCI; Dr Mccabe) of the National Institutes of Health (NIH)
Letters Conflict of Interest Disclosures: All authors have completed and submitted the 6. Centers for Disease Control and Prevention. TBI data and statistics: ICMJE Form for Disclosure of Potential Conflicts of Interest and none were emergency department visits, hospitalizations and deaths, 2001–2010. reported. https://www.cdc.gov/traumaticbraininjury/data/index.html. Accessed Funding/Support: This work was supported by research grants L40DA042452 December 15, 2016. (Dr Veliz), R01DA031160, and R01DA036541 (both Dr McCabe) from the National Institute on Drug Abuse (NIDA), K23HD078502 from the National Institute of Child Health and Human Development (Dr Eckner), and The Heritability of Autism Spectrum Disorder R01CA203809 from the National Cancer Institute (NCI; Dr McCabe) of the National Institutes of Health (NIH). The Monitoring the Future data were Studies have found that autism spectrum disorder (ASD) ag- collected under grant R01DA001411 from NIDA (Dr Schulenberg). gregates in families, and twin studies estimate the propor- Role of the Funder/Sponsor: The NIH had no involvement in the design and tion of the phenotype variance due to genetic factors (herita- conduct of the study; collection, management, analysis, and interpretation of bility) to be about 90%.1 the data; preparation, review, or approval of the manuscript; and decision to In a previous study,2 ASD heritability was estimated to be submit the manuscript for publication. 0.50, and shared familial environmental influences to be 0.04. Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, NCI, or the NIH. To define presence or absence of ASD, the study used a data 1. -

Hypercholesterolemia Effect on Potassium Channels
We are IntechOpen, the world’s leading publisher of Open Access books Built by scientists, for scientists 5,400 134,000 165M Open access books available International authors and editors Downloads Our authors are among the 154 TOP 1% 12.2% Countries delivered to most cited scientists Contributors from top 500 universities Selection of our books indexed in the Book Citation Index in Web of Science™ Core Collection (BKCI) Interested in publishing with us? Contact [email protected] Numbers displayed above are based on latest data collected. For more information visit www.intechopen.com Chapter 5 Hypercholesterolemia Effect on Potassium Channels Anna N. Bukiya and Avia Rosenhouse-Dantsker Additional information is available at the end of the chapter http://dx.doi.org/10.5772/59761 1. Introduction Cholesterol is a major lipid component of the plasma membrane in mammalian cells consti‐ tuting up to 45 mol % with respect to other lipids [1, 2]. Yet, even a limited increase in blood and/or tissue cholesterol of up to 2-3 fold above the physiological level is cytotoxic [1-3] and is associated with the development of cardiovascular disease [4-6]. The underlying source for the effect of cholesterol on cellular functions is its ability to alter the function of multiple membrane proteins including ion channels (see, for example, reviews [7-9]). In recent years, high cholesterol diet has been shown to affect the function of multiple ion channels. In this chapter we focus on the effect of dietary-induced increase in blood and tissue cholesterol levels on potassium channels. Potassium channels are among the largest and most complex types of ion channels.