Metabolism of Carveol and Dihydrocarveol in Rhodococcus Erythropolis DCL14
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.Microorganisms Screening for Limonene Oxidation
Ciência e Tecnologia de Alimentos ISSN: 0101-2061 [email protected] Sociedade Brasileira de Ciência e Tecnologia de Alimentos Brasil LERIN, Lindomar; TONIAZZO, Geciane; de OLIVEIRA, Débora; ROTTAVA, Leda; DARIVA, Cláudio; CANSIAN, Rogério Luis; TREICHEL, Helen; PADILHA, Francine; Ceva ANTUNES, Octávio Augusto Microorganisms screening for limonene oxidation Ciência e Tecnologia de Alimentos, vol. 30, núm. 2, abril-junio, 2010, pp. 399-405 Sociedade Brasileira de Ciência e Tecnologia de Alimentos Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=395940100017 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Ciência e Tecnologia de Alimentos ISSN 0101-2061 Microorganisms screening for limonene oxidation Seleção de microrganismos para oxidação de limoneno Original Lindomar LERIN1, Geciane TONIAZZO2, Débora de OLIVEIRA2*, Leda ROTTAVA1, Cláudio DARIVA3, Rogério Luis CANSIAN2, Helen TREICHEL2, Francine PADILHA3, Octávio Augusto Ceva ANTUNES1 Abstract Limonene is a monoterpene obtained in large amounts from essential oils and is used as a raw material for the synthesis of flavors and fine chemicals. Several pathways or routes for the microbial degradation of limonene making use of the cytochrome P450-dependent monooxygenases have been described. In this study, we present a fermentative screening of microorganisms in order to verify their ability to perform the desirable conversion. In parallel, the PCR technique was used to select the microorganisms that contain the limC gene, which is responsible for the conversion of carveol to carvone. -

Generated by SRI International Pathway Tools Version 25.0, Authors S
Authors: Pallavi Subhraveti Peter D Karp Ingrid Keseler An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Anamika Kothari Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_000223375Cyc: Ketogulonicigenium vulgare WSH-001 (GCF_000223375) Cellular Overview Connections between pathways are omitted for legibility. Ron Caspi a sulfonate a sulfonate L-arabinose L-arabinose L-arabinose L-arabinose L-arabinose spermidine nitrate nitrate phosphate D-galactose D-galactose D-galactose D-galactose D-galactose 3+ 3+ putrescine L-ornithine L-ornithine L-ornithine L-ornithine a sulfonate a sulfonate a sulfonate a sulfonate a sulfonate hydrogencarbonate hydrogencarbonate a sulfonate Fe Fe an amino glycine betaine D-allose D-allose D-allose D-allose D-allose spermidine spermidine spermidine sn-glycerol sn-glycerol spermidine lys lys lys lys molybdate molybdate molybdate molybdate nitrate nitrate nitrate nitrate nitrate nitrate thiosulfate thiosulfate thiosulfate thiosulfate thiosulfate thiosulfate spermidine spermidine ammonium a glycerophosphodiester acid L-carnitine phosphate phosphate phosphate benzoate hydrogencarbonate hydrogencarbonate hydrogencarbonate hydrogencarbonate hydrogencarbonate hydrogencarbonate -

Biosynthesis of New Alpha-Bisabolol Derivatives Through a Synthetic Biology Approach Arthur Sarrade-Loucheur
Biosynthesis of new alpha-bisabolol derivatives through a synthetic biology approach Arthur Sarrade-Loucheur To cite this version: Arthur Sarrade-Loucheur. Biosynthesis of new alpha-bisabolol derivatives through a synthetic biology approach. Biochemistry, Molecular Biology. INSA de Toulouse, 2020. English. NNT : 2020ISAT0003. tel-02976811 HAL Id: tel-02976811 https://tel.archives-ouvertes.fr/tel-02976811 Submitted on 23 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE En vue de l’obtention du DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE Délivré par l'Institut National des Sciences Appliquées de Toulouse Présentée et soutenue par Arthur SARRADE-LOUCHEUR Le 30 juin 2020 Biosynthèse de nouveaux dérivés de l'α-bisabolol par une approche de biologie synthèse Ecole doctorale : SEVAB - Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingenieries Spécialité : Ingénieries microbienne et enzymatique Unité de recherche : TBI - Toulouse Biotechnology Institute, Bio & Chemical Engineering Thèse dirigée par Gilles TRUAN et Magali REMAUD-SIMEON Jury -

Handbook of Essential Oils: Science, Technology, and Applications
Handbook of ESSENTIAL Science, Technology, OILS and Applications Handbook of ESSENTIAL Science, Technology, OILS and Applications Edited by K. Hüsnü Can Bas¸er Gerhard Buchbauer Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2010 by Taylor and Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number: 978-1-4200-6315-8 (Hardback) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the valid- ity of all materials or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint. Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmitted, or uti- lized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopy- ing, microfilming, and recording, or in any information storage or retrieval system, without written permission from the publishers. -

Novel Approaches for Using Dehydrogenases and Ene-Reductases for Organic Synthesis
Novel approaches for using dehydrogenases and ene-reductases for organic synthesis Serena GARGIULO Novel approaches for using dehydrogenases and ene-reductases for organic synthesis Proefschrift ter verkrijging van de graad van doctor aan de Technische Universiteit Delft, op gezag van de Rector Magnificus prof. ir. K.C.A.M. Luyben, voorzitter van het College voor Promoties, in het openbaar te verdedigen op dinsdag 24 februari 2015 om 10.00 uur door Serena GARGIULO Master of Science in Molecular and Industrial Biotechnology van Università degli Studi di Napoli “Federico II”, Italië, geboren te Napels, Italië Dit proefschrift is goedgekeurd door de promotor: Prof Dr. I.W.C.E. Arends Copromotor Dr.F.Hollmann Samenstelling promotiecommissie: Rector Magnificus, voorzitter Prof Dr. I. W. C. E. Arends Technische Universiteit Delft, promotor Dr. F. Hollmann Technische Universiteit Delft, supervisor Prof. Dr. S. De Vries Technische Universiteit Delft Prof. Dr. R. Piccoli Università degli Studi di Napoli “Federico II” Prof. Dr. W. van Berkel Wageningen Universiteit Prof. Dr. G. Grogan University of York Dr. Stephan Luetz Novartis Pharma Prof. Dr. E. J. R. Sudholter Technische Universiteit Delft, reservelid ISBN 978-94-6108-919-9 The research reported in this thesis was supported by the Marie Curie Initial Training Network BIOTRAINS, financed by the European Union through the 7th Framework People Programme (grant agreement number 238531) Ai miei Angeli, in terra e in cielo, a chi ha creduto in me Table of contents Chapter 1 Introduction……………………………………………………………………………1 Chapter 2 A photoenzymatic system for alcohol oxidation……………………………………..29 Chapter 3 A biocatalytic redox isomerization…………………………………………………...47 Chapter 4 Synthetic nicotinamide cofactors for biocatalytic reduction of activated C=C bonds…………………………………………………………………………...67 Chapter 5 Structure of the alcohol dehydrogenase from Thermus sp . -

Investigating Labdane-Related Diterpenoids Biosynthetic Network In
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2012 Investigating labdane-related diterpenoids biosynthetic network in rice: characterization of cytochromes P450 and short-chain alcohol dehydrogenases/reductase Yisheng Wu Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Biochemistry Commons Recommended Citation Wu, Yisheng, "Investigating labdane-related diterpenoids biosynthetic network in rice: characterization of cytochromes P450 and short-chain alcohol dehydrogenases/reductase" (2012). Graduate Theses and Dissertations. 12680. https://lib.dr.iastate.edu/etd/12680 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Investigating labdane-related diterpenoids biosynthetic network in rice: Characterization of cytochromes P450 and short-chain alcohol dehydrogenases/reductase by Yisheng Wu A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Biochemistry Program of Study Committee: Reuben Peters, Major Professor Gustavo MacIntosh Thomas Bobik Bing Yang Mark Hargrove Iowa State University Ames, Iowa 2012 Copyright © Yisheng Wu , 2012. All -

Pea SAD Short-Chain Dehydrogenase/Reductase Author to Whom All Correspondence Should Be Addressed
Plant Physiology Preview. Published on February 22, 2011, as DOI:10.1104/pp.111.173336 1 Running head: Pea SAD Short-Chain Dehydrogenase/Reductase Author to whom all correspondence should be addressed: Åke Strid Akademin för naturvetenskap och teknik och Centrum för livsvetenskap Örebro universitet SE-70182 Örebro Sweden Telephone: +46-19-303603 E-mail: [email protected] Most appropriate journal research area: Cell Biology 1 Copyright 2011 by the American Society of Plant Biologists 2 The Pisum sativum SAD Short-Chain Dehydrogenase/Reductase: Quinone Reduction, Tissue 1 Distribution, and Heterologous Expression Nikolai Scherbak2, Anneli Ala-Häivälä2, Mikael Brosché2, Nathalie Böwer, Hilja Strid, John R. Gittins3, Elin Grahn, Leif A. Eriksson4, and Åke Strid* Akademin för naturvetenskap och teknik och Centrum för livsvetenskap (N.S., A.A.-H., N.B., E.G., L.A.E., Å.S) and Hälsoakademin och Centrum för livsvetenskap (H.S.), Örebro universitet, S-70182 Örebro, Sweden; Biokemi och biofysik (M.B., J.R.G.), Institutionen för Kemi, Göteborgs universitet, P.O. Box 462, S-405 30 Göteborg, Sweden; Division of Plant Biology (M.B.), Department of Biosciences, University of Helsinki, P.O. Box 65, FIN-00014 Helsinki, Finland, and Institute of Technology, University of Tartu, Nooruse 1, Tartu 50411, Estonia. 2 3 Footnotes: 1This work was supported by Helge Ax:son Johnson’s foundation (N.S.), the Carl Trygger Foundation (Å.S.) and the Faculty for Science and Technology at Örebro University (Å.S.). The Strategic Network for Swedish Plant Biotechnology (SSF) funded J.R.G.’s stay in Sweden with a grant to Å.S. -
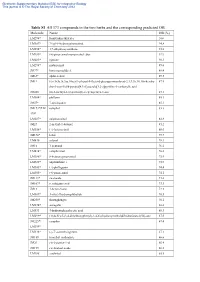
Table S1 All 373 Compounds in the Two Herbs and the Corresponding
Electronic Supplementary Material (ESI) for Integrative Biology This journal is © The Royal Society of Chemistry 2012 Table S1 All 373 compounds in the two herbs and the corresponding predicted OB Molecule Name OB (%) LM294* forsythidmethylester 100 LM387* 7'-epi-8-hydroxypinoresinol 94.8 LM394* 1,7-dihydroxyxanthone 93.8 LM289* (+)-pinoresinol monomethyl ether 92.9 LM285* egenine 90.3 LM298* matairesinol 89.6 JM77* loniceracetalide A 89.4 JM63* alpha-cedrol 89.3 JM1* (-)-(3r,8s,9r,9as,10as)-9-ethenyl-8-(beta-d-glucopyranosyloxy)-2,3,9,9a,10,10a-hexahy 87.5 dro-5-oxo-5h,8h-pyrano[4,3-d]oxazolo[3,2-a]pyridine-3-carboxylic acid JM200 (z)-3-methyl-2-(2-pentenyl)-2-cyclopenten-1-one 87.1 LM304* phillyrin 85.1 JM57* 7-epi-loganin 85.1 JM173*/LM camphol 83.3 398* LM287* epipinoresinol 82.8 JM27 2-methyl-1-butanol 81.2 LM356* (+)-lariciresinol 80.0 JM170* ledol 79.7 LM430 safynol 78.3 JM16 1-pentanol 76.2 LM414* campherenol 76.0 LM386* 8-hydroxypinoresinol 75.9 LM428* onjixanthone i 75.9 LM345* (+)-phillygenin 74.4 LM305* (+)-pinoresinol 74.1 JM113* sweroside 73.6 JM107* secologanic acid 73.3 JM13 1-hexen-3-one 72.3 LM309* 3-ethyl-7hydroxyphthalide 70.5 JM250* shuangkangsu 70.1 LM274* astragalin 68.6 LM331 4-hydroxyphenylacetic acid 68.3 LM299* (3r,4s,5r)-5-(3,4-dimethoxyphenyl)-3,4-bis(hydroxymethyl)dihydrofuran-2(3h)-one 67.5 JM225*/ camphor 67.4 LM399* LM338* (-)-7’-o-methylegenine 67.1 JM189 trimethyl isothiazole 66.6 JM29 cis-2-penten-1-ol 66.4 JM199 cis-linalool oxide 66.2 LM288 erythritol 65.8 Electronic Supplementary Material (ESI) for -

NPC Natural Product Communications
NPC-CMAPSEEC Issue Volume 12. Issue 2. Pages 153-318. 2017 ISSN 1934-578X (printed); ISSN 1555-9475 (online) www.naturalproduct.us NPC Natural Product Communications EDITOR-IN-CHIEF HONORARY EDITOR DR. PAWAN K AGRAWAL PROFESSOR GERALD BLUNDEN Natural Product Inc. The School of Pharmacy & Biomedical Sciences, 7963, Anderson Park Lane, University of Portsmouth, Westerville, Ohio 43081, USA Portsmouth, PO1 2DT U.K. [email protected] [email protected] EDITORS ADVISORY BOARD PROFESSOR ALEJANDRO F. BARRERO Department of Organic Chemistry, University of Granada, Prof. Giovanni Appendino Prof. Niel A. Koorbanally Campus de Fuente Nueva, s/n, 18071, Granada, Spain Novara, Italy Durban, South Africa [email protected] Prof. Norbert Arnold Prof. Chiaki Kuroda PROFESSOR MAURIZIO BRUNO Halle, Germany Tokyo, Japan Department STEBICEF, Prof. Hartmut Laatsch University of Palermo, Viale delle Scienze, Prof. Yoshinori Asakawa Gottingen, Germany Parco d’Orleans II - 90128 Palermo, Italy Tokushima, Japan [email protected] Prof. Marie Lacaille-Dubois Prof. Vassaya Bankova PROFESSOR VLADIMIR I. KALININ Dijon, France Sofia, Bulgaria G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Prof. Shoei-Sheng Lee Far Eastern Branch, Russian Academy of Sciences, Prof. Roberto G. S. Berlinck Taipei, Taiwan Pr. 100-letya Vladivostoka 159, 690022, São Carlos, Brazil Vladivostok, Russian Federation Prof. Anna R. Bilia Prof. M. Soledade C. Pedras [email protected] Saskatoon, Canada Florence, Italy PROFESSOR YOSHIHIRO MIMAKI Prof. Geoffrey Cordell Prof. Luc Pieters School of Pharmacy, Antwerp, Belgium Chicago, IL, USA Tokyo University of Pharmacy and Life Sciences, Prof. Fatih Demirci Prof. Peter Proksch Horinouchi 1432-1, Hachioji, Tokyo 192-0392, Japan Düsseldorf, Germany [email protected] Eskişehir, Turkey Prof. -

Monoterpene Biosynthesis in the Glandulartrichomes Of
Plant Physiol. (1989) 89, 1351-1357 Received for publication July 1, 1988 0032-0889/0000/1351 /07/$01 .00/0 and in revised form December 2, 1988 Biochemical and Histochemical Localization of Monoterpene Biosynthesis in the Glandular Trichomes of Spearmint (Mentha spicata)" 2 Jonathan Gershenzon, Massimo Maffei3, and Rodney Croteau* Institute of Biological Chemistry, Washington State University, Pullman, Washington 99164-6340 ABSTRACT type diterpenes found in the glandular exudate. The head cells The primary monoterpene accumulated in the glandular tri- oftobacco trichomes are the only leafcells able to incorporate chomes of spearmint (Mentha spicata) is the ketone (-)-carvone radiolabeled acetate into duvane diterpenes; removal ofthese which is formed by cyclization of the C10 isoprenoid intermediate cells severely reduces or eliminates duvane production (22). geranyl pyrophosphate to the olefin (-)-limonene, hydroxylation Thus, it is tempting to speculate that all terpenes found in to (-)-trans-carveol and subsequent dehydrogenation. Selective glandular trichomes are synthesized in situ. However, recent extraction of the contents of the glandular trichomes indicated reports of monoterpene synthesis by undifferentiated cells in that essentially all of the cyclase and hydroxylase activities culture (5, 28), coupled to evidence for monoterpene transport resided in these structures, whereas only about 30% of the in intact plants (7, 31), suggest that the site of synthesis may carveol dehydrogenase was located here with the remainder not necessarily be the same as the site of accumulation. located in the rest of the leaf. This distribution of carveol dehy- drogenase activity was confirmed by histochemical methods. Spearmint (Mentha spicata, Lamiaceae) accumulates large Electrophoretic analysis of the partially purified carveol dehydro- quantities of monoterpenes in glandular trichomes, the major genase from extracts of both the glands and the leaves following constituent of which is (-)-carvone (20). -

Frankincense Essential Oil Prepared from Hydrodistillation of Boswellia
Ni et al. BMC Complementary and Alternative Medicine 2012, 12:253 http://www.biomedcentral.com/1472-6882/12/253 RESEARCH ARTICLE Open Access Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model Xiao Ni1, Mahmoud M Suhail2, Qing Yang3, Amy Cao4, Kar-Ming Fung3,5,6, Russell G Postier7, Cole Woolley8, Gary Young8, Jingzhe Zhang1* and Hsueh-Kung Lin3* Abstract Background: Regardless of the availability of therapeutic options, the overall 5-year survival for patients diagnosed with pancreatic cancer remains less than 5%. Gum resins from Boswellia species, also known as frankincense, have been used as a major ingredient in Ayurvedic and Chinese medicine to treat a variety of health-related conditions. Both frankincense chemical extracts and essential oil prepared from Boswellia species gum resins exhibit anti-neoplastic activity, and have been investigated as potential anti-cancer agents. The goals of this study are to identify optimal condition for preparing frankincense essential oil that possesses potent anti-tumor activity, and to evaluate the activity in both cultured human pancreatic cancer cells and a xenograft mouse cancer model. Methods: Boswellia sacra gum resins were hydrodistilled at 78°C; and essential oil distillate fractions were collected at different durations (Fraction I at 0–2 h, Fraction II at 8–10 h, and Fraction III at 11–12 h). Hydrodistillation of the second half of gum resins was performed at 100°C; and distillate was collected at 11–12 h (Fraction IV). Chemical compositions were identified by gas chromatography–mass spectrometry (GC-MS); and total boswellic acids contents were quantified by high-performance liquid chromatography (HPLC). -

Supplementary Information
Supplementary Information The domain composition of sequences from Laurencia dendroidea that were annotated as terpene synthases and presented in Table 2 of the main manuscript were obtained through search for conserved domains using the NCBI Conserved Domain Database (CDD, [1]) and compared with the domain composition of corresponding sequences available in the SwissProt/UniProt and PlantCycDB databases. Analyzing the tables and figures below, we reinforce the Blast annotations presented in Table 2 of the main manuscript text, although biochemical and gene cloning approaches are necessary to prove these in silico identifications. For two of the 21 terpene synthase sequences from Laurencia (identified as nerolidol synthase and (+)-delta-cadinene synthase), we were not able to identify conserved domains, possibly because they were partial sequences. In the case of alpha-bisabolene synthase, we provide the domain composition of a reference sequence and the alignment of the sequence from L. dendroidea with this reference, showing a high similarity between them. Table S1. List of domain hits for a putative (3R)-linalool synthase gene from L. dendroidea. Name Accession Description Interval E-Value DNA repair protein (rad1); All proteins in this rad1 TIGR00596 1–51 1.04e-15 family for which functions are known are ... Figure S1. Conserved domains detected in a putative (3R)-linalool synthase gene from L. dendroidea (edited from the NCBI CDD database, [1]). Table S2. List of domain hits for the (3R)-linalool synthase gene from Zea mays mays (GDQC-116173-MONOMER—PlantCycDB). Name Accession Description Interval E-Value Plant Terpene Cyclases, Class 1; Terpene_cyclase_plant_C1 cd00684 This CD includes a diverse group of 1377–1833 9.57e-107 monomeric plant terpene ..