NPC Natural Product Communications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.Microorganisms Screening for Limonene Oxidation
Ciência e Tecnologia de Alimentos ISSN: 0101-2061 [email protected] Sociedade Brasileira de Ciência e Tecnologia de Alimentos Brasil LERIN, Lindomar; TONIAZZO, Geciane; de OLIVEIRA, Débora; ROTTAVA, Leda; DARIVA, Cláudio; CANSIAN, Rogério Luis; TREICHEL, Helen; PADILHA, Francine; Ceva ANTUNES, Octávio Augusto Microorganisms screening for limonene oxidation Ciência e Tecnologia de Alimentos, vol. 30, núm. 2, abril-junio, 2010, pp. 399-405 Sociedade Brasileira de Ciência e Tecnologia de Alimentos Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=395940100017 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Ciência e Tecnologia de Alimentos ISSN 0101-2061 Microorganisms screening for limonene oxidation Seleção de microrganismos para oxidação de limoneno Original Lindomar LERIN1, Geciane TONIAZZO2, Débora de OLIVEIRA2*, Leda ROTTAVA1, Cláudio DARIVA3, Rogério Luis CANSIAN2, Helen TREICHEL2, Francine PADILHA3, Octávio Augusto Ceva ANTUNES1 Abstract Limonene is a monoterpene obtained in large amounts from essential oils and is used as a raw material for the synthesis of flavors and fine chemicals. Several pathways or routes for the microbial degradation of limonene making use of the cytochrome P450-dependent monooxygenases have been described. In this study, we present a fermentative screening of microorganisms in order to verify their ability to perform the desirable conversion. In parallel, the PCR technique was used to select the microorganisms that contain the limC gene, which is responsible for the conversion of carveol to carvone. -
![Sub-Theme 01: [SWG] Organization & Time: Organizing in the Nexus Between Short and Distant Futures](https://docslib.b-cdn.net/cover/4984/sub-theme-01-swg-organization-time-organizing-in-the-nexus-between-short-and-distant-futures-104984.webp)
Sub-Theme 01: [SWG] Organization & Time: Organizing in the Nexus Between Short and Distant Futures
Sub-theme 01: [SWG] Organization & Time: Organizing in the Nexus between Short and Distant Futures Convenors: Tima Bansal, Ivey Business School, Western University, Canada [email protected] Tor Hernes, Copenhagen Business School, Denmark, & University of South-Eastern Norway [email protected] Joanna Karmowska, Oxford Brookes University, United Kingdom [email protected] Session I: Thursday, July 04, 11:00 to 12:30, SH-PP - Charter Suite Introduction: The Short in the Distant and Vice Versa Chair: Tor Hernes Maximilian Weis and Patricia Klarner Temporal tensions in organizations: How to address different time horizons Discussant(s): Miriam Feuls Miriam Feuls, Mie Plotnikof and Iben Sandal Stjerne Challenging time(s): Exploring methodological challenges of researching temporality & organizing Discussant(s): Lianne Simonse Session II: Thursday, July 04, 14:00 to 15:30 - Parallel Stream - Parallel Stream A: Temporality in Sustainable Development - Room: SH-PP - Charter Suite Chair: Tima Bansal Dimitra Makri Andersen Time will Tell: Temporal Tensions in NGO – Business Partnerships for Sustainability Discussant(s): Christel Dumas Christel Dumas, Jacob Vermeire and Céline Louche Time and space in sustainable finance Discussant(s): Dimitra Makri Andersen Lianne Simonse and Petra Badke-Schaub Strategic design & time: Framing design roadmapping of long term futures Discussant(s): Maximilian Weis Parallel Stream B: Temporal Work and Organizational Performance - Room: SH-PP - Lower Ground Suite Chair: Joanna Karmowska Gerry McGivern, Sue Dopson, Ewan Ferlie and Michael D. Fischer ‘This isn’t the dream you have sold us’: Events, temporal work and expectations in a genetics network Discussant(s): Daniel Z. Mack and Quy Huy Sara Melo How adopting a long-term performance management perspective influences performance measurement, organisational learning, and performance Discussant(s): Gerry McGivern Daniel Z. -

Population Genetics and Disease Predisposing Genes
Abstracts S22 Population Genetics and Disease Predisposing Genes 33 34 HLA CLASS I AND CHEMOKINE GENE EXPRESSION A NEW HLA-DRB1 ALLELE: DRB1* 1152 IN RENAL CELL CARCINOMAS Giuseppina Ozzella, Palmina I. Monaco, Angela Iacona, Elide Calcagni, José María Romero, José Manuel Cózar, Julia Cantón, Antonio Garrido, Claudio Cortini, Daniela Piancatelli, Anna Aureli, Giuseppe Tufano, Teresa Cabrera, Pilar Jiménez, Susana Pedrinaci, Miguel Tallada, Antonina Piazza and Domenico Adorno. Federico Garrido, Francisco Ruiz-Cabello. Servicio Análisis Clínicos e C.N.R. Institute for Organ Transplantation, Rome - Italy Inmunologia, Hospital Universitario Virgen de las Nieves. Granada. Spain Searching for a potential related bone marrow donor, a new HLA- DRB1*11 allele, DRB1*1152, was identifi ed in three members of a Moroccan Berber Immune mechanisms have been suggested to play a role in the natural family. The doubtful presence of a new allele raised from some discrepancies disease course of RCC. The objective of this study was to examine factors among family members’ DRB1 low resolution typing. So, all members were that may be involved in the signifi cant survival benefi t of immunotherapy studied performing PCR SSP high resolution typing. Father’s DRB1 typing for RCC patients. A low frequency of total or HLA haplotype loss was surely permitted to assign DRB1*1104. It was not possible to defi ne the second found and, in parallel, the tumour tissue expressed more HLA classI/ allele because of an ambiguity between DRB1*1117 and some DRB1*14 alleles, B2m mRNA. These data signifi cantly differ from those reported in other due to a false positive reaction. -

Clear Spring Health HMO Plan Provider Directory
Clear Spring Health HMO Plan Provider Directory This directory is current as of December 1, 2019. This directory provides a list of Clear Spring Health’s current network providers. This directory is for the Illinois Service Area: Boone, Clinton, Cook, Du Page, Kane, Kankakee, La Salle, Macoupin, Madison, Mc Henry, Ogle, St. Clair, Stephenson, Will and Winnebago county. To access Clear Spring Health’s online provider directory, you can visit www.clearspringhealthcare.com. For any questions about the information contained in this directory, please call our Member Service Department at 877-384-1241, we are open 8:00 am to 8:00 pm Md on ay – Friday from April 1 – September 30 and 8:00 am to 8:00 pm Monday – Sunday from October 1 – March 31. TTY users should call 711. Out-of-network/non-contracted providers are under no obligation to treat Clear Spring Health members, except in emergency situations. Please call our Member Service number or see your Evidence of Coverage for more information, including the cost-sharing that applies to out-of- network services. Our plan has people and free interpreter services available to answer questions from disabled and non-English speaking members. We can also give you information in Braille, in large print, or other alternate formats at no cost if you need it. We are required to give you information about the plan’s benefits in a format that is accessible and appropriate for you. To get information from us in a way that works for you, please call Member Services or contact Office for Civil Rights. -

Deutsche Gesellschaft Für Experimentelle Und Klinische Pharmakologie Und Toxikologie E.V
Naunyn-Schmiedeberg´s Arch Pharmacol (2013 ) 386 (Suppl 1):S1–S104 D OI 10.1007/s00210-013-0832-9 Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie e.V. Abstracts of the 79 th Annual Meeting March 5 – 7, 2013 Halle/Saale, Germany This supplement was not sponsored by outside commercial interests. It was funded entirely by the publisher. 123 S2 S3 001 003 Multitarget approach in the treatment of gastroesophagel reflux disease – Nucleoside Diphosphate Kinase B is a Novel Receptor-independent Activator of comparison of a proton-pump inhibitor with STW 5 G-protein Signaling in Clinical and Experimental Atrial Fibrillation Abdel-Aziz H.1,2, Khayyal M. T.3, Kelber O.2, Weiser D.2, Ulrich-Merzenich G.4 Abu-Taha I.1, Voigt N.1, Nattel S.2, Wieland T.3, Dobrev D.1 1Inst. of Pharmaceutical & Medicinal Chemistry, University of Münster Pharmacology, 1Universität Duisburg-Essen Institut für Pharmakologie, Hufelandstr. 55, 45122 Essen, Hittorfstr 58-62, 48149 Münster, Germany Germany 2Steigerwald Arzneimittelwerk Wissenschaft, Havelstr 5, 64295 Darmstadt, Germany 2McGill University Montreal Heart Institute, 3655 Promenade Sir-William-Osler, Montréal 3Faculty of Pharmacy, Cairo University Pharmacology, Cairo Egypt Québec H3G 1Y6, Canada 4Medizinische Poliklinik, University of Bonn, Wilhelmstr. 35-37, 53111 Bonn, Germany 3Medizinische Fakultät Mannheim der Universität Heidelberg Institutes für Experimentelle und Klinische Pharmakologie und Toxikologie, Maybachstr. 14, 68169 Gastroesophageal reflux disease (GERD) was the most common GI-diagnosis (8.9 Mannheim, Germany million visits) in the US in 2012 (1). Proton pump inhibitors (PPI) are presently the mainstay of therapy, but in up to 40% of the patients complete symptom control fails. -
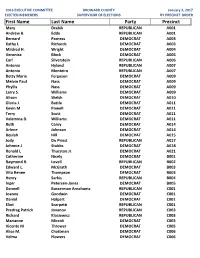
First Name Last Name Party Precinct Mary Drabik REPUBLICAN A001 Andrew B
2016 EXECUTIVE COMMITTEE BROWARD COUNTY January 3, 2017 ELECTED MEMEBERS SUPERVISOR OF ELECTIONS BY PRECINCT ORDER First Name Last Name Party Precinct Mary Drabik REPUBLICAN A001 Andrew B. Eddy REPUBLICAN A001 Bernard Parness DEMOCRAT A003 Kathy L Richards DEMOCRAT A003 Mildred H. Wright DEMOCRAT A004 Veronica Block DEMOCRAT A005 Carl Silverstein REPUBLICAN A006 Antonia Hyland REPUBLICAN A007 Antonio Monteiro REPUBLICAN A007 Betty Marie Ferguson DEMOCRAT A009 Melvin Paul Nass DEMOCRAT A009 Phyllis Nass DEMOCRAT A009 Larry S. Williams DEMOCRAT A009 Alison Welsh DEMOCRAT A010 Gloria J. Battle DEMOCRAT A011 Kevin M Powell DEMOCRAT A011 Terry Scott DEMOCRAT A011 Velemina D. Williams DEMOCRAT A011 Ruth Carey DEMOCRAT A014 Arlene Johnson DEMOCRAT A014 Beulah Hill DEMOCRAT A015 Jody Du Priest REPUBLICAN A017 Johnnie J Stubbs DEMOCRAT A018 Ronald L Thurston Jr. DEMOCRAT A021 Catherine Nicely DEMOCRAT B001 Raymond R. Lovell REPUBLICAN B002 Edward L. McGrath DEMOCRAT B003 Vita Renee Thompson DEMOCRAT B003 Henry Sarkis REPUBLICAN B004 Inger Petersen-Jones DEMOCRAT B005 Dannell Bosserman Anschuetz REPUBLICAN C001 Joanne Goodwin DEMOCRAT C001 Daniel Halpert DEMOCRAT C001 Eliot Scarpetti REPUBLICAN C001 Predrag Patrick Jovanov REPUBLICAN C003 Richard Klosiewicz REPUBLICAN C003 Marianne Miccoli DEMOCRAT C003 Vicente M Thrower DEMOCRAT C005 Alice M. Chattman DEMOCRAT C006 Velma Flowers DEMOCRAT C006 2016 EXECUTIVE COMMITTEE BROWARD COUNTY January 3, 2017 ELECTED MEMEBERS SUPERVISOR OF ELECTIONS BY PRECINCT ORDER David F Booth REPUBLICAN C010 Kathy Mantz REPUBLICAN C011 Robert J. Tersch REPUBLICAN C012 Gary E. Bitner DEMOCRAT C014 Maggie Davidson DEMOCRAT C016 Allison Pollio REPUBLICAN C016 Angela "Angie" Hill REPUBLICAN C017 James Lansing DEMOCRAT C017 Linda Polsney REPUBLICAN C017 Carol Spitler DEMOCRAT C018 Joanne Sterner DEMOCRAT C021 Isabela Dorneles DEMOCRAT C022 David Nelson Altermatt DEMOCRAT C023 Daniel P. -

Biosynthesis of New Alpha-Bisabolol Derivatives Through a Synthetic Biology Approach Arthur Sarrade-Loucheur
Biosynthesis of new alpha-bisabolol derivatives through a synthetic biology approach Arthur Sarrade-Loucheur To cite this version: Arthur Sarrade-Loucheur. Biosynthesis of new alpha-bisabolol derivatives through a synthetic biology approach. Biochemistry, Molecular Biology. INSA de Toulouse, 2020. English. NNT : 2020ISAT0003. tel-02976811 HAL Id: tel-02976811 https://tel.archives-ouvertes.fr/tel-02976811 Submitted on 23 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE En vue de l’obtention du DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE Délivré par l'Institut National des Sciences Appliquées de Toulouse Présentée et soutenue par Arthur SARRADE-LOUCHEUR Le 30 juin 2020 Biosynthèse de nouveaux dérivés de l'α-bisabolol par une approche de biologie synthèse Ecole doctorale : SEVAB - Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingenieries Spécialité : Ingénieries microbienne et enzymatique Unité de recherche : TBI - Toulouse Biotechnology Institute, Bio & Chemical Engineering Thèse dirigée par Gilles TRUAN et Magali REMAUD-SIMEON Jury -

Som-1282 Som-1282 Base En-23431 1..1
ISSN 1725-2423 Official Journal C 178 A of the European Union ★ ★ ★ ★★ ★★ ★★ ★ ★ ★ Volume 53 English edition Information and Notices 3 July 2010 Notice No Contents Page V Announcements ADMINISTRATIVE PROCEDURES European Personnel Selection Office (EPSO) 2010/C 178 A/01 Reserve lists — Open competitions — EPSO/AD/144/09 — Public health — EPSO/AD/145/09 — Food safety — EPSO/AD/146/09 — Food safety — Administrators (AD 5) of Bulgarian, Cypriot, Czech, Estonian, Hungarian, Latvian, Lithuanian, Maltese, Polish, Romanian, Slovak and Slovenian citi zenship in the field of public health ......................................................................................... 1 2010/C 178 A/02 Reserve lists — Open competitions — EPSO/AD/149/09 — Economics — EPSO/AD/150/09 — Micro economics/Business administration — EPSO/AD/151/09 — Audit — Administrators (AD 5) (BG/RO) 4 2010/C 178 A/03 Reserve lists — Open competitions — EPSO/AST/80/09 — HU — EPSO/AST/86/09 — SK — Assis tants (AST 1) in the secretarial field ......................................................................................... 9 EN Price: EUR 3 3.7.2010 EN Official Journal of the European Union C 178 A/1 V (Announcements) ADMINISTRATIVE PROCEDURES EUROPEAN PERSONNEL SELECTION OFFICE (EPSO) RESERVE LISTS (1) OPEN COMPETITIONS EPSO/AD/144/09 — PUBLIC HEALTH EPSO/AD/145/09 — FOOD SAFETY EPSO/AD/146/09 — FOOD SAFETY ADMINISTRATORS (AD 5) of Bulgarian, Cypriot, Czech, Estonian, Hungarian, Latvian, Lithuanian, Maltese, Polish, Romanian, Slovak and Slovenian citizenship IN THE FIELD OF PUBLIC HEALTH (2010/C 178 A/01) EPSO/AD/144/09 — Public health Merit group 1 GRIVA Miroslav JURCZAK Katarzyna PASSANTE Lara Grazia PODNIECE Zinta SCHWARZ Andrea (SISKA) BURIAN Ioana-Raluca SPASSOVA Svetlana Merit group 2 BALOGH Attila BOYADJIEVA Lora CAPLANUSI Mircea Adrian GORANOVA Boriana HOJNY Arkadiusz HORKA Hana IACOB Simona KAJTAR Nora KLCOVA Silvia KRTICKOVA Michaela (1) A successful candidate may explicitly request her/his name not be published. -

UNIVERSIDADE FEDERAL DOS VALES DO JEQUITINHONHA E MUCURI Programa De Pós Graduação Em Ciência Florestal
UNIVERSIDADE FEDERAL DOS VALES DO JEQUITINHONHA E MUCURI Programa de Pós Graduação em Ciência Florestal Any Caroliny Pinto Rodrigues PERFIL DE EXPRESSÃO GÊNICA EM HÍBRIDOS DE Eucalyptus grandis x Eucalyptus urophylla AFETADOS PELO DISTÚRBIO FISIOLÓGICO DO EUCALIPTO (DFE) Diamantina 2020 Any Caroliny Pinto Rodrigues PERFIL DE EXPRESSÃO GÊNICA EM HÍBRIDOS DE Eucalyptus grandis x Eucalyptus urophylla AFETADOS PELO DISTÚRBIO FISIOLÓGICO DO EUCALIPTO (DFE) Tese apresentada à Universidade Federal dos Vales do Jequitinhonha e Mucuri, como parte das exigências do Programa de Pós Graduação em Ciência Florestal, área de concentração em Recursos Florestais, para obtenção do título de “Doutor”. Orientador: Prof. Dr. Marcelo Luiz de Laia Diamantina 2020 Aos meus pais e ao meu irmão por tudo que representam em minha vida e, de maneira mais especial, pelos últimos meses! Dedico com amor e gratidão! AGRADECIMENTOS Agradeço a Deus por ter sempre iluminado e guiado o meu caminho, por ter me dado fé e coragem para seguir a caminhada. Aos meus pais, Marly e Joaquim, os grandes mestres da minha vida! Agradeço o amor, apoio, incentivo e confiança incondicionais. Ao meu irmão, Thiago, que é o meu companheiro, cúmplice e melhor amigo. Agradeço por tornar sempre os meus dias mais alegres e por ter sido força quando eu mais precisei. À minha cunhada, por todo o suporte, força, ajuda e carinho que foram essenciais. A toda a minha família, em especial meus tios Marley, Flávio, Jorge e Mara, que são minha grande fonte de inspiração. A meus eternos amigos Laís (Grazy) e Luiz Paulo (Peré) que foram e são verdadeiros anjos em minha vida. -

Intégration Des Modèles in Vitro Dans La Stratégie D'évaluation De La
Intégration des modèles in vitro dans la stratégie d’évaluation de la sensibilisation cutanée : 2018SACLS003 Thèse de doctorat de l'Université Paris-Saclay NNT préparée à l’Université Paris-Sud École doctorale n°569 Innovation thérapeutique : du fondamental à l'appliqué Spécialité de doctorat: Immunotoxicologie Thèse présentée et soutenue à Chatenay-Malabry, le 26 Janvier 2018, par Elodie Clouet Composition du Jury : Dr. Bernard Maillère Président Directeur de Recherche, CEA (Immunochimie de la réponse immunitaire cellulaire) Pr. Armelle Baeza Rapporteur Professeur des Universités, Paris Diderot (BFA UMR CNRS 8251) Dr. Patricia Rousselle Rapporteur Directeur de Recherche, IBCP Lyon (FRE 3310, CNRS) Dr. Elena Giménez-Arnau Examinateur Chargé de Recherche, Université de Strasbourg (UMR 7177) Dr. Hervé Groux Examinateur Directeur de recherche, Immunosearch Pr. Saadia Kerdine-Römer Directrice de thèse Professeur des Universités, Université Paris-Sud (INSERM UMR-S 996) Dr. Pierre-Jacques Ferret Co-Encadrant Directeur de la Toxicologie et de la Cosmétovigilance, Pierre Fabre SOMMAIRE LISTE DES FIGURES .................................................................................................................................. 3 LISTE DES TABLEAUX ............................................................................................................................... 5 LISTE DES ABREVIATIONS ....................................................................................................................... 6 AVANT-PROPOS ..................................................................................................................................... -

Untersuchungen Zur Regulation Der Polyphenolbiosynthese in Der Erdbeerfrucht (Fragaria Ananassa) Mittels Metabolite Profiling
TECHNISCHE UNIVERSITÄT MÜNCHEN Fachgebiet Biotechnologie der Naturstoffe Untersuchungen zur Regulation der Polyphenolbiosynthese in der Erdbeerfrucht (Fragaria ananassa) mittels Metabolite Profiling Ludwig F. M. Ring Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzende: Univ.-Prof. Dr. B. Poppenberger Prüfer der Dissertation: 1. Univ.-Prof. Dr. W. Schwab 2. Univ.-Prof. Dr. Th. Hofmann 3. Univ.-Prof. Dr. D. R. Treutter Die Dissertation wurde am 17.06.2013 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 22.10.2013 angenommen. „… and all the pieces matter“ Lester Freamon, 2002 meiner Familie Danksagung I Danksagung Meinem Doktorvater Prof. Dr. Wilfried Schwab gilt mein besonderer Dank für die Überlassung des Themas und die Möglichkeit an seinem Fachgebiet zu promovieren. Außerdem danke ich ihm für seine immerwährende Unterstützung und seinen ausstrahlenden Optimismus. Bei Prof. Dr. Brigitte Poppenberger, Prof. Dr. Thomas Hofmann und Prof. Dr. Dieter Treutter bedanke ich mich für die Mitarbeit in der Prüfungskommission. Allen Kooperationspartnern des FraGenomics-Projekts, insbesondere Prof. Dr. Juan Muñoz- Blanco, Dr. Beatrice Denoyes-Rothan und Dr. Amparo Monfort, möchte ich für die gute Zusammenarbeit und die fruchtbaren Diskussionen bei den Projekttreffen danken. Prof. Dr. Victoriano Valpuesta danke ich sehr für die Möglichkeit meine Arbeiten zur Proteinanalytik am Department für Molekularbiologie und Biochemie der Universität Málaga durchführen zu können. Seinem gesamten Arbeitskreis danke ich für die herzliche Aufnahme! Gracias a todos los miembros del grupo! Además, les agradesco a Dra. -

NSF Engineering Research Center for Biorenewable Chemicals, Third Year Renewal Proposal, Volume II NSF Engineering Research Center for Biorenewable Chemicals
NSF Engineering Research Center for Biorenewable Center for Biorenewable Chemicals Annual Reports Chemicals 4-7-2011 NSF Engineering Research Center for Biorenewable Chemicals, Third Year Renewal Proposal, Volume II NSF Engineering Research Center for Biorenewable Chemicals Follow this and additional works at: http://lib.dr.iastate.edu/cbirc_annualreports Part of the Biomedical Engineering and Bioengineering Commons, and the Chemical Engineering Commons Recommended Citation NSF Engineering Research Center for Biorenewable Chemicals, "NSF Engineering Research Center for Biorenewable Chemicals, Third Year Renewal Proposal, Volume II" (2011). Center for Biorenewable Chemicals Annual Reports. 5. http://lib.dr.iastate.edu/cbirc_annualreports/5 This Book is brought to you for free and open access by the NSF Engineering Research Center for Biorenewable Chemicals at Iowa State University Digital Repository. It has been accepted for inclusion in Center for Biorenewable Chemicals Annual Reports by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NSF Engineering Research Center for Biorenewable Chemicals Transforming the THIRD YEAR RENEWALchemical PROPOSALindustry for a sustainable future VOLUME II April 7, 2011 Dr. Brent Shanks, Director Dr. Basil Nikolau, Deputy Director Core Partner Institutions Iowa State University (Lead) Rice University University of California, Irvine University of New Mexico University of Virginia University of Wisconsin Transforming the chemical