Life History and Population Dynamics of Cerambycidae. Chapter 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Genus Canidia Thomson, 1857 (Coleoptera: Cerambycidae, Lamiinae, Acanthocinini)
Zootaxa 927: 1–27 (2005) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 927 Copyright © 2005 Magnolia Press ISSN 1175-5334 (online edition) The genus Canidia Thomson, 1857 (Coleoptera: Cerambycidae, Lamiinae, Acanthocinini) JAMES E. WAPPES¹ & STEVEN W. LINGAFELTER² ¹ American Coleoptera Museum, 179 Fall Creek, Bulverde, TX 78163 U. S. A. [email protected] ² Systematic Entomology Lab, Plant Sciences Institute, Agriculture Research Service, USDA, National Museum of Natural History, Smithsonian Institution, MRC-168, Washington, DC 20013-7012 U. S. A. [email protected] Abstract The lamiine genus Canidia Thomson is redefined with Canidiopsis Dillon and Pseudocanidia Dil- lon as new synonyms. Three new species from Mexico are described and illustrated: Canidia chemsaki, C. giesberti, and C. turnbowi. The following new synonymies are proposed: Canidiop- sis similis Dillon, 1955 and Canidiopsis hebes Dillon, 1955 = Canidia mexicana Thomson, 1860; Pseudocanidia cuernavacae Dillon, 1955 = Dectes spinicornis Bates, 1881; and Dectes (Canidia) balteata var. inapicalis Tippmann, 1960 = Dectes balteatus Lacordaire, 1872. A key to the eight species and one subspecies is presented. Key words: Insecta, Coleoptera, Cerambycidae, Lamiinae, Acanthocinini, Canidia, Dectes, Can- idiopsis, Pseudocanidia, new species, key Resumen: Se redefine el género Canidia Thomson con Canidiopsis Dillon y Pseudocanidia Dillon como sinónimos nuevos. Describimos e ilustramos tres especies nuevas de México: Canidia chemsaki, C. giesberti y C. turnbowi. Se proponen los siguientes sinónimos nuevos: Canidiopsis similis Dillon, 1955 y Canidiopsis hebes Dillon 1955 = Canidia mexicana Thomson, 1860; Pseudocanidia cuernavacae Dillon, 1955 = Dectes spinicornis Bates, 1881; y Dectes (Canidia) balteata inapicalis Tippmann, 1960 = Dectes balteatus Lacordaire, 1872. Se incluye una clave para separar las ocho especies y una subespecie. -

Artificial Laboratory Breeding of Xylophagous Insect Larvae and Its Application in Cytogenetic Studies 2)
Eos, t. LXII, págs. 7-22 (1986). Artificial laboratory breeding of xylophagous insect larvae and its application in cytogenetic studies 2) BY J. R. BARAGAÑO, A. NOTARIO y M. G. DE VIEDMA. INTRODUCTION HAYDAK, in 1936, managed to rear Oryzaephilus surinantensis (L.) in the la- boratory using an artificial diet. Many researchers have followed in his footsteps, so that since then, approximately 260 species of Coleoptera have been raised on nonnatural diets. Among these species there are 121 which are eminently xylophagous. They belong to seven families (Buprestidae, Elateridae, Bostrychiclae, Lyctidae, Myc- teridae, Cerambyciclae and Curculionidae). Their importance, from the economic point of view, varies widely : some of them attack living trees making them a pest ; others feed on dead or decaying wood so that they may be considered harmless or even beneficial (for example in the decomposition of tree stumps in forests) ; finally, a few cause damage to seasoned timber. Therefore, specialists in artificial breeding have been motivated by different objectives, and so have chosen the insect or insects in each case which were most suitable for obtaining specific desired results. It is clear that in the majority of cases the choice was not made at random. Generally, the insect studied was either recently established as a pest or well documented as such. •With these laboratory breeding experiments it is possible on the one hand to draw conclusions about the insects' nutritive requirements, parasitism, ethology etc ; and on the other to obtain enough specimens to try out different phytosanitary treatments with them. Both of these achievements are applicable to effectiye control of the insect problem. -

4 Reproductive Biology of Cerambycids
4 Reproductive Biology of Cerambycids Lawrence M. Hanks University of Illinois at Urbana-Champaign Urbana, Illinois Qiao Wang Massey University Palmerston North, New Zealand CONTENTS 4.1 Introduction .................................................................................................................................. 133 4.2 Phenology of Adults ..................................................................................................................... 134 4.3 Diet of Adults ............................................................................................................................... 138 4.4 Location of Host Plants and Mates .............................................................................................. 138 4.5 Recognition of Mates ................................................................................................................... 140 4.6 Copulation .................................................................................................................................... 141 4.7 Larval Host Plants, Oviposition Behavior, and Larval Development .......................................... 142 4.8 Mating Strategy ............................................................................................................................ 144 4.9 Conclusion .................................................................................................................................... 148 Acknowledgments ................................................................................................................................. -

Title: the Role of Nature Reserves in Preserving Saproxylic Biodiversity: Using Longhorn Beetles (Coleoptera: Cerambycidae) As Bioindicators
Title: The role of nature reserves in preserving saproxylic biodiversity: using longhorn beetles (Coleoptera: Cerambycidae) as bioindicators Author: Lech Karpiński, István Maák, Piotr Węgierek Citation style: Karpiński Lech, Maák István, Węgierek Piotr. (2021). The role of nature reserves in preserving saproxylic biodiversity: using longhorn beetles (Coleoptera: Cerambycidae) as bioindicators. "The European Zoological Journal" (2021, iss. 1, s. 487-504), doi 10.1080/24750263.2021.1900427 The European Zoological Journal, 2021, 487–504 https://doi.org/10.1080/24750263.2021.1900427 The role of nature reserves in preserving saproxylic biodiversity: using longhorn beetles (Coleoptera: Cerambycidae) as bioindicators L. KARPIŃSKI 1*, I. MAÁK 2, & P. WEGIEREK 3 1Museum and Institute of Zoology, Polish Academy of Sciences, Warsaw, Poland, 2Department of Ecology, University of Szeged, Szeged, Hungary, and 3Institute of Biology, Biotechnology and Environmental Protection, Faculty of Natural Sciences, University of Silesia in Katowice, Katowice, Poland (Received 9 August 2020; accepted 2 March 2021) Abstract The potential of forest nature reserves as refuges for biodiversity seems to be overlooked probably due to their small size. These, however, may constitute important safe havens for saproxylic organisms since forest reserves are relatively numerous in Europe. Saproxylic beetles are among the key groups for the assessment of biodiversity in forest habitats and longhorn beetles may play an important role in bioindication as they are ecologically associated with various micro- habitats and considered a very heterogeneous family of insects. To study the role of forest reserves as important habitats for saproxylic beetles, we compared cerambycid assemblages in corresponding pairs of sites (nature reserves and managed stands) in a forest region under high anthropogenic pressure (Upper Silesia, Poland, Central Europe). -

Ethanol and (–)-A-Pinene: Attractant Kairomones for Some Large Wood-Boring Beetles in Southeastern USA
J Chem Ecol (2006) DOI 10.1007/s10886-006-9037-8 Ethanol and (–)-a-Pinene: Attractant Kairomones for Some Large Wood-Boring Beetles in Southeastern USA Daniel R. Miller Received: 12 September 2005 /Revised: 12 December 2005 /Accepted: 2 January 2006 # Springer Science + Business Media, Inc. 2006 Abstract Ethanol and a-pinene were tested as attractants for large wood-boring pine beetles in Alabama, Florida, Georgia, North Carolina, and South Carolina in 2002–2004. Multiple-funnel traps baited with (j)-a-pinene (released at about 2 g/d at 25–28-C) were attractive to the following Cerambycidae: Acanthocinus nodosus, A. obsoletus, Arhopalus rusticus nubilus, Asemum striatum, Monochamus titillator, Prionus pocularis, Xylotrechus integer, and X. sagittatus sagittatus. Buprestis lineata (Buprestidae), Alaus myops (Elateridae), and Hylobius pales and Pachylobius picivorus (Curculionidae) were also attracted to traps baited with (j)-a-pinene. In many locations, ethanol synergized attraction of the cerambycids Acanthocinus nodosus, A. obsoletus, Arhopalus r. nubilus, Monochamus titillator, and Xylotrechus s. sagittatus (but not Asemum striatum, Prionus pocularis,orXylotrechus integer)to traps baited with (j)-a-pinene. Similarly, attraction of Alaus myops, Hylobius pales, and Pachylobius picivorus (but not Buprestis lineata) to traps baited with (j)-a- pinene was synergized by ethanol. These results provide support for the use of traps baited with ethanol and (j)-a-pinene to detect and monitor common large wood- boring beetles from the southeastern region of the USA at ports-of-entry in other countries, as well as forested areas in the USA. Keywords Cerambycidae . Xylotrechus . Monochamus . Acanthocinus Curculionidae . Hylobius . Pachylobius . Elateridae . Alaus . Ethanol a-Pinene . -

25Th U.S. Department of Agriculture Interagency Research Forum On
US Department of Agriculture Forest FHTET- 2014-01 Service December 2014 On the cover Vincent D’Amico for providing the cover artwork, “…and uphill both ways” CAUTION: PESTICIDES Pesticide Precautionary Statement This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish or other wildlife--if they are not handled or applied properly. Use all pesticides selectively and carefully. Follow recommended practices for the disposal of surplus pesticides and pesticide containers. Product Disclaimer Reference herein to any specific commercial products, processes, or service by trade name, trademark, manufacturer, or otherwise does not constitute or imply its endorsement, recom- mendation, or favoring by the United States government. The views and opinions of wuthors expressed herein do not necessarily reflect those of the United States government, and shall not be used for advertising or product endorsement purposes. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, D.C. -

Boring Beetles (Coleoptera: Scolytidae, Buprestidae, Cerambycidae) in White Spruce (Picea Glauca (Moench) Voss) Ecosystems of Alaska
United States Department of Agriculture Effect of Ecosystem Disturbance Forest Service on Diversity of Bark and Wood- Pacific Northwest Research Station Boring Beetles (Coleoptera: Research Paper PNW-RP-546 April 2002 Scolytidae, Buprestidae, Cerambycidae) in White Spruce (Picea glauca (Moench) Voss) Ecosystems of Alaska Richard A. Werner This publication reports research involving pesticides. It does not contain recommenda- tions for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate state and federal agencies, or both, before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish or other wildlife—if they are not handled or applied properly. Use all pesticides selectively and carefully. Follow recommended practices for the disposal of surplus pesticides and pesticide containers. Author Richard A. Werner was a research entomologist (retired), Pacific Northwest Research Station, 8080 NW Ridgewood Drive, Corvallis, OR 97330. He is currently a volunteer at the Pacific Northwest Research Station conducting research for the Long Term Ecological Research Program in Alaska. Abstract Werner, Richard A. 2002. Effect of ecosystem disturbance on diversity of bark and wood-boring beetles (Coleoptera: Scolytidae, Buprestidae, Cerambycidae) in white spruce (Picea glauca (Moench) Voss) ecosystems of Alaska. Res. Pap. PNW-RP-546. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 15 p. Fire and timber harvest are the two major disturbances that alter forest ecosystems in interior Alaska. Both types of disturbance provide habitats that attract wood borers and bark beetles the first year after the disturbance, but populations then decrease to levels below those in undisturbed sites. -

Bioseries 06 2007.Pdf
Invasive alien flora and fauna in South Africa: expertise and bibliography by Charles F. Musil & Ian A.W. Macdonald Pretoria 2007 SANBI Biodiversity Series The South African National Biodiversity Institute (SANBI) was established on 1 September 2004 through the signing into force of the National Environmental Management: Biodiversity Act (NEMBA) No. 10 of 2004 by President Thabo Mbeki. The Act expands the mandate of the former National Botanical Institute to include responsibilities relating to the full diversity of South Africa’s fauna and flora, and builds on the internationally respected programmes in conservation, research, education and visitor services developed by the National Botanical Institute and its predecessors over the past century. The vision of SANBI is to be the leading institution in biodiversity science in Africa, facilitating conservation, sustainable use of living resources, and human wellbeing. SANBI’s mission is to promote the sustainable use, conservation, appreciation and enjoyment of the exceptionally rich biodiversity of South Africa, for the benefit of all people. SANBI Biodiversity Series publishes occasional reports on projects, technologies, workshops, symposia and other activities initiated by or executed in partnership with SANBI. Technical editor: Gerrit Germishuizen and Emsie du Plessis Design & layout: Daleen Maree Cover design: Sandra Turck The authors: C.F. Musil—Senior Specialist Scientist, Global Change & Biodiversity Program, South African National Biodiversity Institute, Private Bag X7, Claremont, 7735 ([email protected]) I.A.W. Macdonald—Extraordinary Professor, Sustainability Institute, School of Public Management and Planning, Stellenbosch University ([email protected]) How to cite this publication MUSIL, C.F. & MACDONALD, I.A.W. 2007. Invasive alien flora and fauna in South Africa: expertise and bibliography. -
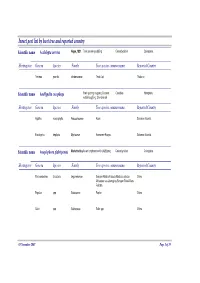
Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae -
Deadwood and Saproxylic Beetle Diversity in Naturally Disturbed and Managed Spruce Forests in Nova Scotia
A peer-reviewed open-access journal ZooKeysDeadwood 22: 309–340 and (2009) saproxylic beetle diversity in disturbed and managed spruce forests in Nova Scotia 309 doi: 10.3897/zookeys.22.144 RESEARCH ARTICLE www.pensoftonline.net/zookeys Launched to accelerate biodiversity research Deadwood and saproxylic beetle diversity in naturally disturbed and managed spruce forests in Nova Scotia DeLancey J. Bishop1,4, Christopher G. Majka2, Søren Bondrup-Nielsen3, Stewart B. Peck1 1 Department of Biology, Carleton University, Ottawa, Ontario, Canada 2 c/o Nova Scotia Museum, 1747 Summer St., Halifax, Nova Scotia Canada 3 Department of Biology, Acadia University, Wolfville, Nova Scotia, Canada 4 RR 5, Canning, Nova Scotia, Canada Corresponding author: Christopher G. Majka ([email protected]) Academic editor: Jan Klimaszewski | Received 26 March 2009 | Accepted 6 April 2009 | Published 28 September 2009 Citation: Bishop DJ, Majka CG, Bondrup-Nielsen S, Peck SB (2009) Deadwood and saproxylic beetle diversity in naturally disturbed and managed spruce forests in Nova Scotia In: Majka CG, Klimaszewski J (Eds) Biodiversity, Bio- systematics, and Ecology of Canadian Coleoptera II. ZooKeys 22: 309–340. doi: 10.3897/zookeys.22.144 Abstract Even-age industrial forestry practices may alter communities of native species. Th us, identifying coarse patterns of species diversity in industrial forests and understanding how and why these patterns diff er from those in naturally disturbed forests can play an essential role in attempts to modify forestry practices to minimize their impacts on native species. Th is study compares diversity patterns of deadwood habitat structure and saproxylic beetle species in spruce forests with natural disturbance histories (wind and fi re) and human disturbance histories (clearcutting and clearcutting with thinning). -

Your Name Here
RELATIONSHIPS BETWEEN DEAD WOOD AND ARTHROPODS IN THE SOUTHEASTERN UNITED STATES by MICHAEL DARRAGH ULYSHEN (Under the Direction of James L. Hanula) ABSTRACT The importance of dead wood to maintaining forest diversity is now widely recognized. However, the habitat associations and sensitivities of many species associated with dead wood remain unknown, making it difficult to develop conservation plans for managed forests. The purpose of this research, conducted on the upper coastal plain of South Carolina, was to better understand the relationships between dead wood and arthropods in the southeastern United States. In a comparison of forest types, more beetle species emerged from logs collected in upland pine-dominated stands than in bottomland hardwood forests. This difference was most pronounced for Quercus nigra L., a species of tree uncommon in upland forests. In a comparison of wood postures, more beetle species emerged from logs than from snags, but a number of species appear to be dependent on snags including several canopy specialists. In a study of saproxylic beetle succession, species richness peaked within the first year of death and declined steadily thereafter. However, a number of species appear to be dependent on highly decayed logs, underscoring the importance of protecting wood at all stages of decay. In a study comparing litter-dwelling arthropod abundance at different distances from dead wood, arthropods were more abundant near dead wood than away from it. In another study, ground- dwelling arthropods and saproxylic beetles were little affected by large-scale manipulations of dead wood in upland pine-dominated forests, possibly due to the suitability of the forests surrounding the plots. -

On the Occurence of Five Pyrophilous Beetle Species in the Swiss Central Alps (Leuk, Canton Valais)
On the occurence of five pyrophilous beetle species in the Swiss Central Alps (Leuk, canton Valais) Autor(en): Pradella, Cinzia / Wermelinger, Beat / Obrist, Martin K. Objekttyp: Article Zeitschrift: Mitteilungen der Schweizerischen Entomologischen Gesellschaft = Bulletin de la Société Entomologique Suisse = Journal of the Swiss Entomological Society Band (Jahr): 83 (2010) Heft 3-4 PDF erstellt am: 10.10.2021 Persistenter Link: http://doi.org/10.5169/seals-403008 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte Dritter, die über dieses Angebot zugänglich sind. Ein Dienst der ETH-Bibliothek ETH Zürich, Rämistrasse 101, 8092 Zürich, Schweiz, www.library.ethz.ch http://www.e-periodica.ch MITTEILUNGEN DER SCHWEIZERISCHEN ENTOMOLOGISCHEN GESELLSCHAFT BULLETIN DE LA SOCIÉTÉ ENTOMOLOGIQUE SUISSE 83: 187-197,2010 On the occurrence of five pyrophilous beetle species in the Swiss Central Alps (Leuk, Canton Valais) Cinzia Pradella1, Beat Wermelinger2, Martin K.