Background Material: 1997-11-12 Chlorinated Fluorocarbons As
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
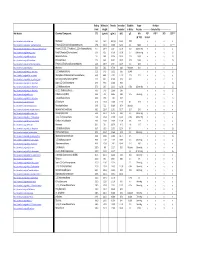
Detectable Compounds by MIP.Pdf
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

Dichlorodifluoromethane Dcf
DICHLORODIFLUOROMETHANE DCF CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: 99.5% (vol.) Common Synonyms Gas Colorless Faint odor Not flammable 7.2 Storage Temperature: Ambient Arcton 6 4.2 Flammable Limits in Air: Not flammable Eskimon 12 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Not F-12 Visible vapor cloud is produced. 7.4 Venting: Safety relief flammable Freon 12 7.5 IMO Pollution Category: Currently not available Frigen 12 4.4 Fire Extinguishing Agents Not to Be Genetron 12 Used: Not flammable 7.6 Ship Type: Currently not available Halon 122 4.5 Special Hazards of Combustion 7.7 Barge Hull Type: 3 Isotron 12 Products: Although nonflammable, Ucon 12 dissociation products generated in a fire may be irritating or toxic. 8. HAZARD CLASSIFICATIONS Notify local health and pollution control agencies. 4.6 Behavior in Fire: Helps extinguish fire. 8.1 49 CFR Category: Nonflammable gas Avoid inhalation. 4.7 Auto Ignition Temperature: Not 8.2 49 CFR Class: 2.2 flammable 8.3 49 CFR Package Group: Not pertinent. Not flammable. 4.8 Electrical Hazards: Not pertinent Fire Cool exposed containers with water. 8.4 Marine Pollutant: No 4.9 Burning Rate: Not flammable 8.5 NFPA Hazard Classification: Not listed 4.10 Adiabatic Flame Temperature: Currently 8.6 EPA Reportable Quantity: 5000 pounds CALL FOR MEDICAL AID. not available Exposure 8.7 EPA Pollution Category: D 4.11 Stoichometric Air to Fuel Ratio: Not VAPOR 8.8 RCRA Waste Number: U075 Not irritating to eyes, nose or throat. -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

SAFETY DATA SHEET Halocarbon R-503
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 007306 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Refer to manufacturer or supplier for information on recovery or recycling. Hazards not otherwise : Liquid can cause burns similar to frostbite. -

Refrigerant Safety Refrigerant History
Refrigerant Safety The risks associated with the use of refrigerants in refrigeration and airconditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment. Refrigerant History Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926. With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable. The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants. -

Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards
A111D3 TTbS?? o z C/J NBS SPECIAL PUBLICATION 260-108 o ^EAU U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard Reference Materials: Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards QC 100 U57 R. Mavrodineanu and T. E. Gills 260-108 1987 m he National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal i s t0 strengthen and advance the nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research to assure international competitiveness and leadership of U.S. industry, science arid technology. NBS work involves development and transfer of measurements, standards and related science and technology, in support of continually improving U.S. productivity, product quality and reliability, innovation and underlying science and engineering. The Bureau's technical work is performed by the National Measurement Laboratory, the National Engineering Laboratory, the Institute for Computer Sciences and Technology, and the Institute for Materials Science and Engineering. The National Measurement Laboratory Provides the national system of physical and chemical measurement; • Basic Standards 2 coordinates the system with measurement systems of other nations and • Radiation Research furnishes essential services leading to accurate and uniform physical and • Chemical Physics chemical measurement throughout the Nation's scientific community, • Analytical Chemistry industry, and commerce; provides advisory and research services to other Government agencies; conducts physical and chemical research; develops, produces, and distributes Standard Reference Materials; provides calibration services; and manages the National Standard Reference Data System. -

Flammable Refrigerants Firefighter Training
Flammable refrigerants firefighter training: Hazard assessment and demonstrative testing FINAL REPORT BY: Noah L. Ryder, P. E. Stephen J. Jordan Fire & Risk Alliance, LLC. Rockville, Maryland, USA. Peter B. Sunderland, Ph.D. University of Maryland Department of Fire Protection Engineering College Park, Maryland, USA. May 2019 © 2019 Fire Protection Research Foundation 1 Batterymarch Park, Quincy, MA 02169-7417, USA Email: [email protected] | Web: nfpa.org/foundation ---page intentionally left blank--- —— Page ii —— FOREWORD The ongoing push toward sustainability of refrigeration systems will require the adoption of low global warming potential (GWP) refrigerants to meet the shift in environmental regulations. Fire safety is a lingering issue with the new age of flammable refrigerants being adopted and first responders may not be familiar with the change in material hazards or the appropriate response procedures required to safely handle these fire scenarios. This project is part of the overall two-year project with a goal to enhance firefighter safety and reduce potential injury by providing training on the hazards from appliances with flammable refrigerants. It will document the information about flammable refrigerants technologies and the hazards to emergency responders and develop interactive training modules to transfer the knowledge to the fire service. This report is focused to develop material documenting the hazards associated with flammable refrigerant technologies and the risks posed to the first responders. The material documentation included a literature review, Task 1, identifying baseline information on flammable refrigerants, their existing usage and implementation into products, potential integration into future technologies, and finally any existing guidance and best practices on response and tactics. -

"Fluorine Compounds, Organic," In: Ullmann's Encyclopedia Of
Article No : a11_349 Fluorine Compounds, Organic GU¨ NTER SIEGEMUND, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany WERNER SCHWERTFEGER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany ANDREW FEIRING, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States BRUCE SMART, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States FRED BEHR, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States HERWARD VOGEL, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States BLAINE MCKUSICK, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States 1. Introduction....................... 444 8. Fluorinated Carboxylic Acids and 2. Production Processes ................ 445 Fluorinated Alkanesulfonic Acids ...... 470 2.1. Substitution of Hydrogen............. 445 8.1. Fluorinated Carboxylic Acids ......... 470 2.2. Halogen – Fluorine Exchange ......... 446 8.1.1. Fluorinated Acetic Acids .............. 470 2.3. Synthesis from Fluorinated Synthons ... 447 8.1.2. Long-Chain Perfluorocarboxylic Acids .... 470 2.4. Addition of Hydrogen Fluoride to 8.1.3. Fluorinated Dicarboxylic Acids ......... 472 Unsaturated Bonds ................. 447 8.1.4. Tetrafluoroethylene – Perfluorovinyl Ether 2.5. Miscellaneous Methods .............. 447 Copolymers with Carboxylic Acid Groups . 472 2.6. Purification and Analysis ............. 447 8.2. Fluorinated Alkanesulfonic Acids ...... 472 3. Fluorinated Alkanes................. 448 8.2.1. Perfluoroalkanesulfonic Acids -

Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

Product Stewardship Summary Carbon Tetrachloride
Product Stewardship Summary Carbon Tetrachloride May 31, 2017 version Summary This Product Stewardship Summary is intended to give general information about Carbon Tetrachloride. It is not intended to provide an in-depth discussion of all health and safety information about the product or to replace any required regulatory communications. Carbon tetrachloride is a colorless liquid with a sweet smell that can be detected at low levels; however, odor is not a reliable indicator that occupational exposure levels have not been exceeded. Its chemical formula is CC14. Most emissive uses of carbon tetrachloride, a “Class I” ozone-depleting substance (ODS), have been phased out under the Montreal Protocol on Substances that Deplete the Ozone Layer (Montreal Protocol). Certain industrial uses (e.g. feedstock/intermediate, essential laboratory and analytical procedures, and process agent) are allowed under the Montreal Protocol. 1. Chemical Identity Name: carbon tetrachloride Synonyms: tetrachloromethane, methane tetrachloride, perchloromethane, benzinoform Chemical Abstracts Service (CAS) number: 56-23-5 Chemical Formula: CC14 Molecular Weight: 153.8 Carbon tetrachloride is a colorless liquid. It has a sweet odor, and is practically nonflammable at ambient temperatures. 2. Production Carbon tetrachloride can be manufactured by the chlorination of methyl chloride according to the following reaction: CH3C1+ 3C12 —> CC14 + 3HC1 Carbon tetrachloride can also be formed as a byproduct of the manufacture of chloromethanes or perchloroethylene. OxyChem manufactures carbon tetrachloride at facilities in Geismar, Louisiana and Wichita, Kansas. Page 1 of 7 3. Uses The use of carbon tetrachloride in consumer products is banned by the Consumer Product Safety Commission (CPSC), under the Federal Hazardous Substance Act (FHSA) 16 CFR 1500.17. -

Refrigerants
Related Commercial Resources CHAPTER 29 REFRIGERANTS Refrigerant Properties .................................................................................................................. 29.1 Refrigerant Performance .............................................................................................................. 29.6 Safety ............................................................................................................................................. 29.6 Leak Detection .............................................................................................................................. 29.6 Effect on Construction Materials .................................................................................................. 29.9 EFRIGERANTS are the working fluids in refrigeration, air- cataracts, and impaired immune systems. It also can damage sensi- R conditioning, and heat-pumping systems. They absorb heat tive crops, reduce crop yields, and stress marine phytoplankton (and from one area, such as an air-conditioned space, and reject it into thus human food supplies from the oceans). In addition, exposure to another, such as outdoors, usually through evaporation and conden- UV radiation degrades plastics and wood. sation, respectively. These phase changes occur both in absorption Stratospheric ozone depletion has been linked to the presence of and mechanical vapor compression systems, but not in systems oper- chlorine and bromine in the stratosphere. Chemicals with long ating on a gas cycle using a -

The Search for Alternative Refrigerants -- a Molecular Approach
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Purdue E-Pubs Purdue University Purdue e-Pubs International Refrigeration and Air Conditioning School of Mechanical Engineering Conference 1988 The eS arch for Alternative Refrigerants -- A Molecular Approach M. O. McLinden National Bureau of Standards D. A. Didion National Bureau of Standards Follow this and additional works at: http://docs.lib.purdue.edu/iracc McLinden, M. O. and Didion, D. A., "The eS arch for Alternative Refrigerants -- A Molecular Approach" (1988). International Refrigeration and Air Conditioning Conference. Paper 69. http://docs.lib.purdue.edu/iracc/69 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Complete proceedings may be acquired in print and on CD-ROM directly from the Ray W. Herrick Laboratories at https://engineering.purdue.edu/ Herrick/Events/orderlit.html THE SEARCH FOR ALTERNATIVE REFRIGERANTS- -A MOLECULAR APPROACH Mark 0. McLinden and David A. Didion National Bureau of Standards Gaithersburg, MD 20899 ABSTRACT The impending prodllction limitations on the fully halogenated CFG refrigerants is causing an intense search for possible substitutes. This study reviews the historical development of the CFG refrigerants and the relationship between molecular structure and t:he ozone depletio11 and greenhouse warming potentials. It then demonstrates that the halogenated hydrocarbons (a class of compounds