A) Cerebral Cortex: • Outer Most Gray Matter of Cerebrum, Covers Hemispheres , Named Pallium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Evaluation of Hydrocephalus
591 MR Evaluation of Hydrocephalus Taher EI Gammal1 An analysis of sagittal T1-weighted MR studies was performed in 23 patients with Marshall B. Allen, Jr.2 hydrocephalus, 58 patients with atrophy, and 100 normal patients. The average rna mil Betty Sue Brooks 1 lopontine distance was 1.15 cm for the normal group, 1.2 cm for patients with atrophy, Edward K. Mark2 and 7.5 mm for patients with hydrocephalus. A reduction of the mamillopontine distance below 1.0 cm was found in 22 patients with hydrocephalus,S patients with atrophy, and 15 normal patients. Dilatation of the anterior third ventricle was noted in 21 patients in the hydrocephalus group and in none of the patients in the atrophy and normal groups. The average thickness of the corpus callosum at the level of the foramen of Monro was 6 mm in normal subjects and was reduced below 6 mm in 16 of the hydrocephalus patients. Smooth elevation of the corpus callosum was noted in 20 hydrocephalus patients, in 2 patients with atrophy, and in none of the normal patients. MR improves the accuracy of diagnosis in patients with hydrocephalus both because of its ability to show small obstructing lesions that are not depicted by CT and because the mass effect of the distended supratentorial ventricles produces anatomic changes that are delineated with precision by MR. CT provides incomplete information for diagnosing hydrocephalus produced by very small or isodense pathologic changes in the aqueduct and in the foramen of Monro [1, 2]. Even with advanced CT techniques, use of contrast media in the subarachnoid space and ventricular system was essential in the CT evaluation of some cases of hydrocephalus [3-5]. -
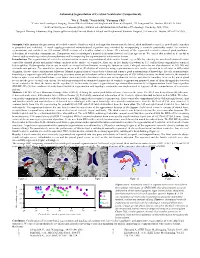
Automated Segmentation of Cerebral Ventricular Compartments
Automated Segmentation of Cerebral Ventricular Compartments 1Wu Y, 2Pohl K, 3Warfield SK, 1Cuttmann CRG. 1Center for Neurological Imaging, Harvard Medical School and Brigham and Women's Hospital, 221 Longwood Av., Boston, MA 02115 USA, 2Artificial Intelligence Laboratory,http://www.ai.mit.edu Massachusetts Institute of Technology, Cambridge MA, USA., 3Surgical Planning Laboratory http://www.spl.harvard.edu Harvard Medical School and Brigham and Women's Hospital, 75 Francis St., Boston, MA 02115 USA, Synopsis: Fully automated segmentation of cerebral ventricle chambers, which is designed to discriminate the lateral, third and fourth ventricles, as well as the aqueduct is presented and validated. A novel topology-restricted intensity-based algorithm was extended by incorporating a ventricle probability model for ventricle segmentation, and validated on 124 coronal SPGR sections of a healthy volunteer’s brain. 3D-rendering of the segmented ventricles showed good qualitative delineation of ventricular compartments. Comparison with a radiologist’s manual delineation showed excellent agreement. We expect this method to be useful in clinical studies involving ventricular morphometry and for improving the segmentation of white matter lesions. Introduction: The segmentation of ventricles is important for accurate segmentation of white matter lesions, e.g. in MS. by reducing the misclassification of lesions caused by choroid plexus and partial volume artifacts at the surface of ventricles. Also, one in five hundred newborn in U.S. suffers from congenital or acquired hydrocephalus. Hydrocephalus also occurs in adults as a result of head trauma, meningitis, tumors or cysts. Enlarged ventricles are also observed in AD, MS and schizophrenia patients. The quantitative measurement, as well as 3D display of ventricles using segmentation in vivo can be expected to be of value in differential diagnosis, disease characterization and follow-up. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Human Brain – 4 Part 1313104
V1/17-2016© Human Brain – 4 Part 1313104 Left Hemisphere of Cerebrum, Lateral View 1. 37. Cingulate Gyrus a. Motor Area (Somatomotor Cortex) 38. Cingulate Sulcus b. Sensory Area (Somatosensory Cortex) 39. Subfrontal Part of Cingulate Sulcus 2. Frontal Lobe 40. Sulcus of Corpus Callosum 3. Temporal Lobe 41. Body of Corpus Callosum 4. Parietal Lobe 42. Genu of Corpus Callosum 5. Occipital Lobe 43. Splenium of Corpus Callosum 6. Left Hemisphere of Cerebellum 44. Septum Pellucidum 7. Superior Frontal Gyrus 45. Body of Fornix 8. Middle Frontal Gyrus 46. Anterior Commissure 9. Inferior Frontal Gyrus 47. Intermediate Mass 10. Precentral Gyrus (Somatomotor Cortex) 48. Posterior Commissure 11. Postcentral Gyrus (Somatosensory Cortex) 49. Thalamus 12. Superior Parietal Lobule 50. Foramen of Monro 13. Inferior Parietal Lobule 51. Hypothalamic Sulcus 14. Lateral Occipital Gyri 52. Lamina Terminalis 15. Superior Temporal Gyrus 53. Optic Chiasm 16. Middle Temporal Gyrus 54. Pineal Gland 17. Inferior Temporal Gyrus 55. Pineal Recess 18. Superior Frontal Sulcus 56. Corpora Quadrigemina 19. Pre-central Sulcus 57. Sylvian Aqueduct 20. Central Sulcus 58. Superior Medullary Vellum 21. Post-central Sulcus 59. Fourth Ventricle 22. Intraparietal Sulcus 60. Central Canal of Medulla Oblongata 23. Transverse Occipital Sulcus 61. Arbor Vitae 24. Lateral Occipital Sulcus 62. Vermis of Cerebellum 25. Parieto-occipital Sulcus 63. Medulla Oblongata 26. Superior Temporal Sulcus 64. Medial Longitudinal Fasciculus 27. Middle Temporal Sulcus 65. Pons 28. Lateral Cerebral Sulcus (Sylvian Fissure) 66. Longitudinal Fibers of Pons 29. Anterior Horizontal Ramus of Sylvian Fissure 67. Cerebral Peduncle 30. Anterior Ascending Ramus of Sylvian Fissure 68. Tuber Cinereum 31. -

Zebrafish Models of Neurodevelopmental
International Journal of Molecular Sciences Review Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques Raquel Vaz 1 , Wolfgang Hofmeister 2,3 and Anna Lindstrand 4,* 1 Department of Molecular Medicine and Surgery, Center for Molecular Medicine, Karolinska Institutet, 171 76 Stockholm, Sweden; [email protected] 2 Laboratory of Molecular and Cellular Cardiology, Department of Clinical Biochemistry and Pharmacology, Odense University Hospital, 5000 Odense, Denmark; [email protected] 3 Novo Nordisk Foundation for Stem Cell Biology (Danstem), University of Copenhagen, 2200 Copenhagen, Denmark 4 Department of Molecular Medicine and Surgery, Center for Molecular Medicine and Clinical Genetics, Karolinska University Laboratory, Karolinska University Hospital, 171 76 Stockholm, Sweden * Correspondence: [email protected]; Tel.: +46-8-517-765-38 Received: 29 January 2019; Accepted: 11 March 2019; Published: 14 March 2019 Abstract: For the past few years there has been an exponential increase in the use of animal models to confirm the pathogenicity of candidate disease-causing genetic variants found in patients. One such animal model is the zebrafish. Despite being a non-mammalian animal, the zebrafish model has proven its potential in recapitulating the phenotypes of many different human genetic disorders. This review will focus on recent advances in the modeling of neurodevelopmental disorders in zebrafish, covering aspects from early brain development to techniques used for modulating gene expression, as well as how to best characterize the resulting phenotypes. We also review other existing models of neurodevelopmental disorders, and the current efforts in developing and testing compounds with potential therapeutic value. Keywords: zebrafish; neurodevelopmental disorders; disease models; functional assays 1. -

CENTRAL NERVOUS SYSTEM Composed from Spinal Cord and Brain
CENTRAL NERVOUS SYSTEM composed from spinal cord and brain SPINAL CORD − is developmentally the oldest part of CNS − is long about 45 cm in adult − fills upper 2/3 of vertebral canal cranial border at level of: foramen magnum, pyramidal decussation, exit of first pair of spinal nerves caudal border: level of L1 vertebra – medullary cone – filum terminale made by pia mater (ends at level of S2 vertebra) – spinal roots below L1 vertebra form cauda equina two enlargements: • cervical enlargement (CV – ThI): origin of nerves for upper extremity – brachial plexus • lumbosacral enlargement (LI – SII): origin of nerves for lower extremity – lumbosacral plexus Spinal segment is part of spinal cord where 1 pair of spinal n. exits. Spinal cord consists of 31 spinal segments and 31 pairs of spinal nn.: 8 cervical, 12 thoracic, 5 lumbar, 1 coccygeal. Spinal nn. leave spinal cord through íntervertebral foramens. Denticulate ligg. attach spinal segments to vertebral canal. Dermatome is part of skin innervated by 1 spinal nerve. external features: • anterior median fissure • anterolateral sulcus – exits of anterior roots of spinal nn. (laterally to anterior median fissure) • posterolateral sulcus – exits of posterior roots of spinal nn. • posterior median sulcus • posterior intermediate sulcus internal features: White matter • anterior funiculus (between anterior median fissure and anterolateral sulcus) • lateral funiculus (between anterolateral and posterolateral sulci) • posterior funiculus (between posterolateral sulcus and posterior median sulcus) is divided by posterior intermediate sulcus to: − gracile fasciculus – medial one − cuneate fasciculus – lateral one, both for sensory tracts of fine sensation White matter of spinal cord contains fibres of ascending and descending nerve tracts. -

The Ventricles of the Brain
The ventricles of the brain The ventricles These are fluid filled cavities within the brain that represent extension of the central canal of the spinal cord into the brain and consist of four chambers that are filled with cerebrospinal fluid (CSF) ( liquor cerebrospinallis ). They are lined by ependymal cells which are one type of glial cells and are responsible for choroid plexus formation which in turn responsible for CSF production. They are responsible for production, transport and removal of the CSF. The functions of the CSF 1) Protection : act as a cushion to the brain to limit neural damage in head injuries 2) Buoyancy :The brain being immersed in the CSF and the brain weight is reduced to 25 gm and this protect the base of the brain 3) Chemical stability : a good homeostatic environment created by the CSF for proper functioning of the brain, like low extra cellular K for synaptic transmission The ventricles There are two lateral ventricles one in each cerebral hemisphere and are connected to the unpaired third ventricle via the interventricular foramen (foramen of Monro) and this third ventricle is connected to the forth ventricle via the cerebral aqueduct (Aqueduct of Sylvius) and the CSF then flow out to the subarachnoid apace through three apertures one central called foramen of Magendie and two lateral called foramena of Lushka. The lateral ventricles The two lateral ventricles form the largest cavities of the ventricular system and each one has its own extension, the anterior horn (frontal horn) , inferior horn (temporal horn) and posterior horn (occipital horn). Important structures border the lateral ventricles are the Caudate nucleus and the thalamus. -

Measurement of Brain...Pdf
بسم الله الرحمن الرحيم Sudan University of Sciences & Technology College of Graduated Studies Measurement of Brain Ventricular Dimensions using Magnetic Resonance Imaging قياس أبعاد التجاويف الدماغيه بإستخدام التصوير بالرني النغناطيس A Thesis Submitted for Partial Fulfillment of M.Sc. Degree in Diagnostic Radiology Technology By: Badawi Mustafa Badawi Suliman Supervisor: Dr. Asma Ibrahim Ahmed Assistant professor 2016 الهيه قال تعالى : (يا أَيها ا ّل ِذين آمنوا ِإذا ِقيل لكم تفسحوا ف ِ ي ا ْلمجا ِل ِس فا ْفسحوا َ ّ َ َ َ ُ َ َ َ ُ ْ َ َ ّ ُ َ َ َ َ ُ ي ْفس ِح الّله لكم و ِإذا ِقيل انشوا فانشوا يرف ِع الّله ا ّل ِذين آمنوا ِمنكم وا ّل ِذين َ َ ُ َ ُ ْ َ َ َ ُ ُ َ ُ ُ َ ْ َ ُ َ َ ُ ُ ْ َ َ ( ِ ُأو ُتوا ا ْلع ْل َم َدرَ َجا ٍت َوالّل ُه ِب َما َت ْع َم ُلو َن َخِبيٌ (سورة الجادله(11 2 Dedication To; my mother, the star that lightened my life. To the great teacher who sacrifice all for me and my brothers to help and support, my father. 3 Acknowledgment First of all, I thank Allah the Almighty for helping me complete this project. I thank Dr. Asma Ibrahim Ahmed, my supervisor, for her help and guidance. I would like to express my gratitude to Dr. Mohamed Ahmed Alfaki, Dr. Kmal Alraih Sanhori and the whole staff of Nelien 4 medical diagnostic center and Antalya center for their great help and support. -
Axis Scientific Deluxe Brain A-106198
Axis Scientific Deluxe Brain A-106198 Hypothalamic Pineal Cingulate Paracentral Sagittal View Interventricular foramen sulcus recess sulcus Sagittal View (Right) (Foramen of Monro) lobule (Left) Anterior View Precuneus Cingulate gyrus Body of fornix Interthalamic adhesion Splenium of Body of corpus callosum corpus callosum Superior frontal gyrus Lateral ventricle Cavity of third ventricle Cuneus Sulcus of corpus callosum Calcarine Septum pellucidum Posterior commissure sulcus Genu of corpus callosum Lateral View Pineal Cingulate sulcus (subfrontal) (Right) gland Anterior commissure Sylvian Lamina terminalis Superior medullary aqueduct vellum Optic recess Anterior Corpora Optic chiasm commissure quadrigemina Infundibular recess Vermis of cerebellum Vermis of Tuber cinereum Frontal lobe Pituitary gland cerebellum Pons Mammillary body Oculomotor nerve Arbor vitae Hypoglossal Pons Fourth Interpeduncular nerve (CN XII) (CN Ill) (Cerebellar white ventricle Cerebral fossa matter) peduncle Olive Pyramidal Medulla Longitudinal Decussation tract Vestibulocochlear Central canal of oblongata fibers of pons of pyramids nerve (CN VIII) medulla oblongata Medial longitudinal Flocculus of cerebellum Abducens nerve (CN VI) fasciculus Glossopharyngeal nerve (CN IX) Pyramidal tract Corona radiata Lateral View Posterior Lateral Vagus nerve (CN X) Hypoglossal nerve (CN XII) Inferior parietal (Left) Pre-central Central View (Left) Lateral geniculate body Lentiform Olive Motor area sulcus Post-central lobe lntraparietal Cerebellar tonsil sulcus sulcus nucleus -

Radiologic Features of Septooptic Dysplasia: De Morsier Syndrome
443 Radiologic Features of Septooptic Dysplasia: de Morsier Syndrome John Anthony O'Dwyer1 The clinical and neuroradiologic features in 22 patients with septooptic dysplasia Thomas H. Newton2 are reviewed. The most consistent abnormality seen in 15 patients was absence of the William F. Hoyt3 septum pellucidum and flattening of the roofs and inferior pointing of the floors of the anterior horns of the lateral ventricles. Hypoplasia of the anterior optic pathways was seen in 10 patients and a primitive optic ventricle was seen in two others. All patients had clinical optic disc hypoplasia; 12 patients had pituitary hormonal deficiencies. In most instances the diagnosis of septooptic dysplasia can be established by physical examination but neuroradiologic study is required to document associated structural abnormalities. In 1956, de Morsier [1] coined the term septooptic dysplasia to describe the brain of an adult in whom hypoplasia of the optic discs was associated with agenesis of the septum pellucidum. This midline malformation of the brain includes dysplasia of the anterior third ventricle, persistence of the optic ventricle, agenesis of the septum pellucidum, and agenesis or hypoplasia of the anterior optic pathway and hypothalamus. Occasionally the chiasmal commissure is absent. Growth retardation as well as other endocrine abnormalities are frequent associated conditions [2-9]. We review features of this syndrome which have received little attention in the radiologic literature [8, 10-13]. Materials and Methods The records of 22 patients with septooptic dysplasia were reviewed . The syndrome was considered present only in those patients who had both optic nerve hypoplasia and either Received January 14, 1980; accepted after pituitary / hypothalamic dysfunction or a structural midline brain abnormality. -

Axonogenesis and Morphogenesis in the Embryonic Zebrafish Brain
The Journal of Neuroscience, February 1992, 72(2): 467-462 Axonogenesis and Morphogenesis in the Embryonic Zebrafish Brain Linda S. Ross, Timothy Parrett, and Stephen S. Easter, Jr. Biology Department, University of Michigan, Ann Arbor, Michigan 48109-1048 We have examined early neuronal differentiation and ax- A hallmark of the vertebrate brain is the complex yet precise onogenesis in the fore- and midbrain of zebrafish embryos set of interconnections between neurons; understandingthe de- to address general issues of early vertebrate brain devel- velopmental basisof this complex circuitry hasbeen a challenge opment. AChE expression and HNK-1 antibody immuno- to embryologistsfor decades.Most contemporary studies,such reactivity were used as markers for differentiated neurons as those of the retinotectal pathway, have examined relatively and axons, respectively. late phasesof ontogeny, long after early circuits have been es- The pattern of neuronal differentiation followed a stereo- tablished by the early outgrowth of pioneer fibers (Easter and typed sequence. AChE-positive cells first appeared between Taylor, 1989). The development of the earliest tracts used to 14 and 16 hr in three small, isolated, bilaterally symmetrical be a major concern of developmental neurobiologists,especially clusters on the surface of the brain. The three clusters-the Coghill (19 13, 1929), Tello (1923), Windle (1932) and Henick dorsorostral, ventrorostral, and ventrocaudal clusters- (1938). However, the techniquesavailable to them were limited, proved to be -

Filling Patterns in Contrast Ventriculography
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.37.4.449 on 1 April 1974. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1974, 37, 449-454 Filling patterns in contrast ventriculography LAWRENCE FERGUSON' From the Division of Neurosurgery, Institute of Neurological Sciences, Southern General Hospital, Glasgow SYN OPSIS The filling patterns of a negative contrast material (air), a positive contrast water soluble material (Conray), and a positive contrast water insoluble material (Myodil) were examined in 60 normal ventriculograms. Using a scoring system developed for this study, Conray was found effective for outlining the ipsilateral (injected) lateral ventricle, the third ventricle, the aqueduct of Sylvius, and the fourth ventricle. Air was the most effective for the noninjected lateral ventricle, while Myodil was best for A-P demonstration of the aqueduct. The clinical implications of these findings are discussed. Now that angiography is the basic neuroradio- ate or meglucamine iothalamate 60% had been logical investigation in most clinics, ventriculo- injected through a frontal burr hole in every case Protected by copyright. graphy is more commonly reserved for accurate except one, where a parietal approach was used. A definition of specific regions either for localizing scoring system was devised for the lateral ventricle, third ventricle, aqueduct of Sylvius, and fourth lesions or identifying anatomical landmarks ventricle to minimize the influence of the imagina- during stereotactic studies. To be successful the tive eye (Table 1). examination must outline the desired area reliably while remaining safe for the patient. There is now available a variety of contrast 1 media: negative contrast, usually air, or positive TABLE contrast, which may be either in oily solution SCORING SYSTEM iophendylate (Myodil) or aqueous solution meglucamine iothalamate 60% (Conray).