Effects of a Mixture of Ivy Leaf Extract and Coptidis Rhizome on Patients with Chronic Bronchitis and Bronchiectasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Drug Information Sheet("Kusuri-No-Shiori")
Drug Information Sheet("Kusuri-no-Shiori") Internal Published: 05/2017 The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment. Brand name:PRANLUKAST TABLETS 112.5mg "CEO" Active ingredient:Pranlukast hydrate Dosage form:white to pale yellow tablet, diameter: 7.5 mm, thickness: 2.7 mm Print on wrapping:(face) プランルカスト 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg (back) PRANLUKAST 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg Effects of this medicine This medicine selectively binds to leukotriene receptor and inhibits its action. It consequently suppresses increase in airway contraction, vascular permeability, mucosal edema and hypersensitivity. It is usually used to treat bronchial asthma and allergic rhinitis. However, it cannot stop the attack of bronchial asthma already in progress but prevents the asthma attack. Before using this medicine, be sure to tell your doctor and pharmacist ・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines. ・If you are pregnant or breastfeeding. ・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.) Dosing schedule (How to take this medicine) ・Your dosing schedule prescribed by your doctor is(( to be written by a healthcare professional)) ・In general, for adults, take 2 tablets (225 mg of the active ingredient) at a time, twice a day after breakfast and dinner. -

Inhibitory Activity of Pranlukast and Montelukas Against Histamine
Showa Univ J Med Sci 21(2), 77~84, June 2009 Original Inhibitory Activity of Pranlukast and Montelukas Against Histamine Release and LTC4 Production from Human Basophils 1, 2 1 1 Satoshi HIBINO ), Ryoko ITO ), Taeru KITABAYASHI ), 1 2 Kazuo ITAHASHI ) and Toshio NAKADATE ) Abstract : Leukotriene receptor antagonists(LTRAs)are routinely used to treat bronchial asthma and are thought to act mostly by inhibiting leukotriene receptors. However, there is no preclinical or clinical evidence of the direct effect of LTRAs on histamine release from and leukotriene(LT)C4 produc- tion by basophils. We used anti-IgE antibody(Ab), FMLP, and C5a to induce histamine release, and anti-IgE Ab and FMLP to stimulate LTC 4 production. Basophils were exposed to different concentrations of pranlukast and montelu- kast, and then to anti-IgE Ab, FMLP, and C5a. Culture supernatant histamine and LTC 4 levels were measured by using a histamine ELISA kit and a LTC 4 EIA kit, respectively. Histamine release was expressed as a percentage of the total histamine content(%HR)induced by anti-IgE Ab, FMLP, or C5a. To evaluate the effects of pranlukast and montelukast on histamine release and LTC 4 production, we calculated the percent inhibition of histamine release and LTC 4 production, expressed as percent inhibition, at different concentrations of pranlukast and montelukast. Pranlukast significantly inhibited histamine release stimulated by FMLP and C5a, but had no effect on histamine release stimulated by anti-IgE Ab. By comparison, montelukast signicantly inhibited histamine release stimulated by FMLP, C5a, and anti-IgE Ab, in a concentration-dependent manner. Both pranlukast and montelukast signicantly inhibited LTC 4 production stimulated by anti-IgE Ab and FMLP. -

A PARTNER for CHANGE the Asia Foundation in Korea 1954-2017 a PARTNER Characterizing 60 Years of Continuous Operations of Any Organization Is an Ambitious Task
SIX DECADES OF THE ASIA FOUNDATION IN KOREA SIX DECADES OF THE ASIA FOUNDATION A PARTNER FOR CHANGE A PARTNER The AsiA Foundation in Korea 1954-2017 A PARTNER Characterizing 60 years of continuous operations of any organization is an ambitious task. Attempting to do so in a nation that has witnessed fundamental and dynamic change is even more challenging. The Asia Foundation is unique among FOR foreign private organizations in Korea in that it has maintained a presence here for more than 60 years, and, throughout, has responded to the tumultuous and vibrant times by adapting to Korea’s own transformation. The achievement of this balance, CHANGE adapting to changing needs and assisting in the preservation of Korean identity while simultaneously responding to regional and global trends, has made The Asia Foundation’s work in SIX DECADES of Korea singular. The AsiA Foundation David Steinberg, Korea Representative 1963-68, 1994-98 in Korea www.asiafoundation.org 서적-표지.indd 1 17. 6. 8. 오전 10:42 서적152X225-2.indd 4 17. 6. 8. 오전 10:37 서적152X225-2.indd 1 17. 6. 8. 오전 10:37 서적152X225-2.indd 2 17. 6. 8. 오전 10:37 A PARTNER FOR CHANGE Six Decades of The Asia Foundation in Korea 1954–2017 Written by Cho Tong-jae Park Tae-jin Edward Reed Edited by Meredith Sumpter John Rieger © 2017 by The Asia Foundation All rights reserved. No part of this book may be reproduced without written permission by The Asia Foundation. 서적152X225-2.indd 1 17. 6. 8. 오전 10:37 서적152X225-2.indd 2 17. -

Drug Class Review Controller Medications for Asthma
Drug Class Review Controller Medications for Asthma Final Update 1 Report April 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Original Report: November 2008 Daniel E. Jonas, MD, MPH Roberta C. M. Wines, MPH Marcy DelMonte, PharmD, BCPS Halle R. Amick, MSPH Tania M. Wilkins, MS Brett D. Einerson, MPH Christine L. Schuler, MD Blake A. Wynia, MPH Betsy Bryant Shilliday, Pharm.D., CDE, CPP Produced by RTI-UNC Evidence-based Practice Center Cecil G. Sheps Center for Health Services Research University of North Carolina at Chapel Hill 725 Martin Luther King Jr. Blvd, CB# 7590 Chapel Hill, NC 27599-7590 Tim Carey, M.D., M.P.H., Director Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2011 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Update 1 Report Drug Effectiveness Review Project The medical literature relating to this topic is scanned periodically. (See http://www.ohsu.edu/xd/research/centers-institutes/evidence-based-policy- center/derp/documents/methods.cfm for description of scanning process). Prior versions of this report can be accessed at the DERP website. -
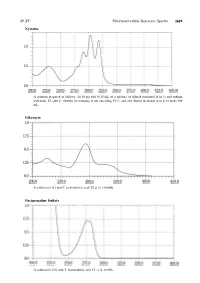
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible -

Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology CLENBUTEROL ELISA KIT INSTRUCTIONS PRODUCT #101219 & 101216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Clenbuterol and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Clenbuterol ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug- enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -

Understanding Interparticle Interactions in Dry Powder Inhalation: Glass Beads As an Innovative Model Carrier System
UNDERSTANDING INTERPARTICLE INTERACTIONS IN DRY POWDER INHALATION: GLASS BEADS AS AN INNOVATIVE MODEL CARRIER SYSTEM DOCTORAL THESIS SUMBITTED IN FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR IN NATURAL SCIENCES AT KIEL UNIVERSITY, GERMANY by Niklas Ludwig Renner Kiel 2017 Referee: Prof. Dr. Regina Scherließ Co-Referee: Prof. Dr. Thomas Kunze Date of Exam: 13.10.2017 Accepted for publication: 13.10.2017 Prof. Dr. Natascha Oppelt (Dean) Published research articles contributing to the present thesis: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler performance Dalby, R.N. (Ed.), RDD Europe 2017 2 (2017), 195-200 Renner, N; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Nano- and microstructured model carrier surfaces to alter dry powder inhaler performance International Journal of Pharmaceutics 518 (2017), 20-28 Conference contributions: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler per- formance Respiratory Drug Delivery, Nice, France (2017) Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. A deeper insight into the impact of chemical surface properties on inhalation perfor- mance Drug Delivery to the Lungs 27, Edinburgh, Scotland (2016) Renner, N.; Scherließ, R.; Steckel, H. Glass beads as model carriers in dry powder inhalers: the influence of chemical sur- face properties on inhalation performance International Congress on Particle Technology, Nurnberg, Germany (2016) Renner, N.; Scherließ, R.; Steckel, H. Investigating the influence of carrier surface roughness on drug delivery in DPIs 10th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Tech- nology, Glasgow, Scotland (2016) Renner, N.; Kutelova, Z.; Scherließ, R.; Steckel, H. -

The Role of 5-Lipoxygenase in Pathophysiology and Management of Neuropathic Pain
REVIEW ARTICLE THE ROLE OF 5-LIPOXYGENASE IN PATHOPHYSIOLOGY AND MANAGEMENT OF NEUROPATHIC PAIN Pascanus Lamsihar PT∗, Faldi Yaputra∗∗, Jimmy FA Barus4 and I Putu Eka Widyadharma∗∗,1 ∗Department of Neurology, Provincial Mental Hospital, West Borneo, Indonesia., ∗∗Department of Neurology, Faculty of Medicine, Udayana University-Sanglah General Hospital, Bali, Indonesia., 4Department of Neurology, Faculty of Medicine, Atma Jaya Catholic University, Jakarta-Indonesia. ABSTRACT Neuropathic pain (NP) is a pain caused by lesions in the nervous system. Several causes of NP are traumatic, metabolic disorders, ischemia, toxins, infections, immune-related, and hereditary. The pathophysiology of NP is very complicated and unknown entirely. Therefore the treatment of NP is still unsatisfactory. Recent studies believed the critical role of primary inflammatory mediators in the pathophysiology of NP especially leukotrienes (LTs). The 5- lipoxygenase enzyme (5-LOX) is an enzyme that plays a role in the metabolism of arachidonic acid into LTs. Leukotrienes (LTs) are the essential inflammatory mediators in the pathophysiology of NP. Leukotriene B4 (LTB4) can cause chemotaxis on neutrophils, lowering nociceptors threshold and may contribute to NP. Several studies believed the administration of 5-LOX inhibitors or LTs receptor antagonists could be useful in the management of NP. The purpose of this review is to summarize the involvement of 5-LOX enzyme as an essential role in the pathophysiology and management of NP. KEYWORDS 5-lipoxygenase, leukotrienes, -

The Role of Pharmacogenomics in Improving the Management of Asthma
The Role of Pharmacogenomics in Improving the Management of Asthma The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kazani S, ME Wechsler, E. Israel. 2010. TThe Role of Pharmacogenomics in Improving the Management of Asthma. Journal of Allergy and Clinical Immunology 125, no. 2: 295–302. Published Version 10.1016/j.jaci.2009.12.014 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:42659246 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP NIH Public Access Author Manuscript J Allergy Clin Immunol. Author manuscript; available in PMC 2011 February 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Allergy Clin Immunol. 2010 February ; 125(2): 295±302. doi:10.1016/j.jaci.2009.12.014. THE ROLE OF PHARMACOGENOMICS IN IMPROVING THE MANAGEMENT OF ASTHMA Shamsah Kazani, M.D. [Instructor in Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Michael E. Wechsler, M.D. [Assistant Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Elliot Israel, M.D. [Associate Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Abstract There is a large amount of interindividual variability in both therapeutic and adverse responses to asthma therapies. Genetic variability may account for 50–60% of this variability. -

Diamandis Thesis
!"!#$ CHEMICAL GENETIC INTERROGATION OF NEURAL STEM CELLS: PHENOTYPE AND FUNCTION OF NEUROTRANSMITTER PATHWAYS IN NORMAL AND BRAIN TUMOUR INITIATING NEURAL PRECUSOR CELLS by Phedias Diamandis A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy. Department of Molecular Genetics University of Toronto © Copyright by Phedias Diamandis 2010 Phenotype and Function of Neurotransmitter Pathways in Normal and Brain Tumor Initiating Neural Precursor Cells Phedias Diamandis Doctor of Philosophy Department of Molecular Genetics University of Toronto 2010 &'(!)&*!% The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain brings promise for the treatment of neurological diseases and has yielded new insight into brain cancer. The complete repertoire of signaling pathways that governs these cells however remains largely uncharacterized. This thesis describes how chemical genetic approaches can be used to probe and better define the operational circuitry of the NSC. I describe the development of a small molecule chemical genetic screen of NSCs that uncovered an unappreciated precursor role of a number of neurotransmitter pathways commonly thought to operate primarily in the mature central nervous system (CNS). Given the similarities between stem cells and cancer, I then translated this knowledge to demonstrate that these neurotransmitter regulatory effects are also conserved within cultures of cancer stem cells. I then provide experimental and epidemiologically support for this hypothesis and suggest that neurotransmitter signals may also regulate the expansion of precursor cells that drive tumor growth in the brain. Specifically, I first evaluate the effects of neurochemicals in mouse models of brain tumors. I then outline a retrospective meta-analysis of brain tumor incidence rates in psychiatric patients presumed to be chronically taking neuromodulators similar to those identified in the initial screen. -

Lamas for COPD Systematic Review
Comparative safety and effectiveness of inhaled long -acting agents (corticosteroids, beta agonists, anticholinergics) for chronic obstructive pulmonary disease Comprehensive Research Plan: Systematic Review Unit April 4th, 2014 Andrea C. Tricco, PhD1 and Sharon E. Straus, MD, MSc1,2 30 Bond Street, Toronto ON, M5B 1W8 www.odprn.ca [email protected] Background Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation in the lungs [1]. COPD is commonly assessed by clinical examination and spirometry. Important indicators considered in the diagnosis of COPD include age over 40 years and any of the following: 1) progressive and persistent dyspnea that worsens with exercise, 2) chronic cough, 3) chronic sputum production, 4) history of exposure to smoke from tobacco or cooking, occupational dusts and chemicals, and 5) family history of COPD [1]. COPD causes significant burden of illness, reduced quality of life, and premature death [2]. Symptoms include chronic cough, sputum production, and dyspnea [3]. The global prevalence of COPD has been estimated at 7.6% using data from a systematic review including 28 countries [4]. However, this is likely a conservative estimate, due to under-reporting and under-diagnosis. The prevalence and burden of COPD is rising due the greater proportion of elderly people in the population [1]. It is estimated that COPD will be the third-leading cause of death by 2020 [5]. The treatment of COPD usually involves reducing exposure (e.g., smoking cessation, occupation modifications), increasing exercise, and implementing appropriate pharmacologic therapy [1]. The most common drug classes are beta2-agonists, anticholinergics, and methylxanthines. Inhaled corticosteroids (ICS) and systemic corticosteroids are often useful for acute exacerbations.