Testing the Mьnch Hypothesis of Long Distance Phloem Transport in Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Book of Abstracts
CHALLENGES FOR PLANT NUTRITION IN CHANGING ENVIRONMENTS International Workshop and Meeting of the German Society of Plant Nutrition 2012 University of Bonn September 5 – 8, 2012 Book of Abstracts Table of Contents Plenary Session: Introductory talks ................................................. 2 Plenary Session S1: Processes on leaf surfaces ................................. 5 Plenary Session S2: Plant water relations .......................................... 14 Plenary Session S3: Nutrient dynamics in changing environments .... 26 Plenary Session S4: Crop responses to nutrient imbalances ............. 38 Plenary Session S5: Phenotyping and early stress responses ........... 80 Poster Session P1: Fertilization (inorganic) ...................................... 92 Poster Session P2: Fertilization (organic) / Soil amendments ........ 110 Poster Session P3: Nutrient efficiency / Genomics ......................... 131 Poster Session P4: Root physiology / Root-soil interactions........... 148 Poster Session P5: Physiological response to abiotic stress .......... 169 Poster Session P6: Physiological response to nutrient imbalances 182 Poster Session P7: Nutrients and ecosystems / Climate change ... 200 Poster Session P8: Signalling / Quality / Phenotyping .................... 215 1 DGP Meeting September 5-9, 2012 Plenary Session: Introductory talks 2 DGP Meeting September 5-9, 2012 Plant nutrition in a changing environment. Patrick H. Brown Department of Plant Sciences, University of California, Davis, CA 95616, US; E-mail: [email protected] The scientific discipline of plant nutrition is wonderfully broad in its scale and its scope. From the exploration of the function of nutrients as signals and regulators of plant function, to the role of plant nutrients in agricultural productivity and food quality, to the exploration of the effects of nutrient losses on global environments, plant nutrition is a truly integrative discipline and will play a critical role in mans’ ability to adapt to environmental change. -

From the Root Surface to the Xylem Elements (3, 5, 11, 20)
THE DIURNAL VARIATION IN THE TRANSLOCATION OF MINERALS ACROSS BEAN ROOTS JOHN B. HANSON' AND ORLIN BIDDULPH DEPARTMENT OF BOTANY, THE STATE COLLEGE OF WASHINGTON, PULLMAN, WASHINGTON Received August 22, 1952 Present knowledge about the mechanism whereby minerals are trans- ferred from the nutrient medium to the root xylem is quite limited. The initial step involves an exchange of hydrogen, hydroxyl, or bicarbonate ions on the protoplasmic surface of the root for nutrient ions of the substrate (13). The amount of any ion gained by such exchange is a function of the rate at which metabolic energy is produced by the root, and, within limits, of the concentration of the ion in the substrate. Absorbed ions appear to enter into labile cytoplasmic complexes which are created or maintained by the expenditure of energy, and which seem to be specific for different ions or groups of related ions. It is now generally held that the protoplasts of the root cells are inter- connected by plasmodesmata forming a symplast of cytoplasm that extends from the root surface to the xylem elements (3, 5, 11, 20). Hence, ions entering the cytoplasmic phase by exchange have a pathway through living material to any of the three aqueous phases-external, vacuolar, or tracheal. The cytoplasm in some manner utilizes energy derived from aerobic respira- tion to speed the distribution of ions throughout the symplast, and to pro- duce high vacuolar and tracheal concentrations of the ions. There is no convincing evidence as to how movement of ions through the cytoplasm occurs, but several theories and speculations on the matter are current. -

AUXIN: TRANSPORT Subject: Botany M.Sc
Saumya Srivastava_ Botany_ MBOTCC-7_Patna University Topic: AUXIN: TRANSPORT Subject: Botany M.Sc. (Semester II), Department of Botany Course: MBOTCC- 7: Physiology and Biochemistry; Unit – III Dr. Saumya Srivastava Assistant Professor, P.G. Department of Botany, Patna University, Patna- 800005 Email id: [email protected] Saumya Srivastava_ Botany_ MBOTCC-7_Patna University Auxin transport The main axes of shoots and roots, along with their branches, exhibit apex–base structural polarity, and this structural polarity has its origin in the polarity of auxin transport. Soon after Went developed the coleoptile curvature test for auxin, it was discovered that IAA moves mainly from the apical to the basal end (basipetally) in excised oat coleoptile sections. This type of unidirectional transport is termed polar transport. Auxin is the only plant growth hormone known to be transported polarly. A significant amount of auxin transport also occurs in the phloem, and this is the principal route by which auxin is transported acropetally (i.e., toward the tip) in the root. Thus, more than one pathway is responsible for the distribution of auxin in the plant. Polar transport is not affected by the orientation of the tissue (at least over short periods of time), so it is independent of gravity. Tissues differ in degree of polarity of IAA transport. In coleoptiles, vegetative stems, and leaf petioles, basipetal transport predominates. Polar transport of auxin in shoots tends to be predominantly basipetal. Acropetal transport here is minimal. In roots, on the other hand, there appear to be two transport streams. An acropetal stream, arriving from the shoot, flows through xylem parenchyma cells in the central cylinder of the root and directs auxin toward the root tip. -

Phloem Transport: Mass Flow Hypothesis
BIOLOGY TRANSPORT IN PLANTS Phloem Transport: Mass Flow Hypothesis Contents Phloem Translocation ................................................................................................................................. 3 Mass Flow Hypothesis ................................................................................................................................ 6 Go to Top www.topperlearning.com 2 BIOLOGY TRANSPORT IN PLANTS Phloem Translocation The organic compounds such as glucose and sucrose produced during photosynthesis are translocated from the green cells to the non-green parts of plants through the phloem tissue. The transport of photosynthates from the leaves to the apices, roots, fruits, buds and tubers of the plant through the phloem is called translocation of organic solutes or long distance transport. Translocation occurs through the phloem in the upward, downward and radial directions from the leaves to the storage organs. The process of translocation requires expenditure of metabolic energy, and the solute moves at the rate of 100 cm/hr. Chemical analysis of the phloem sap reveals the presence of up to 90% sugars such as sucrose, raffinose, stachyose and verbascose. 14 Rabideau and Burr (1945) provided CO2 to a leaf during photosynthesis (Tracer technique). Sugars synthesised in this leaf got labelled with 14C (tracer). The presence of radioactively labelled sugars in the phloem revealed that the solutes are translocated through the phloem. Evidences in Support of Phloem Translocation Some evidences which support that organic solutes are translocated through the phloem: Ringing or Girdling Experiments To determine whether the xylem or phloem tissue is involved in translocation, it is possible to remove the cortex and the phloem of the stem in the form of a girdle. If the xylem is involved in transport, the roots found below the ring should not undergo any kind of modification because the xylem is intact in this experiment. -

Dynamic Function and Regulation of Apoplast in the Plant Body
J. Plant Res. 111: 133-148, 1998 Journal of Plant Research (~) by The Botanical Society of Japan 1998 JPR Symposium Dynamic Function and Regulation of Apoplast in the Plant Body Naoki Sakurai Faculty of Integrated Arts and Sciences, Hiroshima University, Higashi Hiroshima, 739 Japan Apoplast is the internal environment of plant. Our body energy. The direction of two flows is reverse. Usually, the posses the intemal environment that consists of blood, two routes are allotted to xylem vessel and sieve tube. lympha, and tissue fluid. Plant cells are also cultivated and A German plant scientist, E. ML~nch (1930) coined the term surrounded by a liquid medium in the apoplast. As well as apoplast. He termed the water path apoplast, and the other various important functions of the internal environment in part symplast. He noticed that not only xylem vessel but our body, apoplast function is also prerequisite for the plant also cell wall space is the water path and recognized them life. There are so far seven distinct functions of apoplast. as a single continuum of transportation system of water, but (1) Growth regulation with apoplastic enzymes by altering ignored the space for gas exchange. In terms of circulation cell-wall properties through degradation, synthesis, orienta- of mass flow in plant body described above, apoplast should tion and cross-linking of supra molecules of cell walls, such include the air space for gas exchange. Therefore, the as cellulose, non-cellulosic polysaccharides, proteins, and description that plant body consists of apoplast and symplast lignin; (2) Skeleton sustained by cellulose microfibrils, lignin is a simple and clear definition of plant body. -

Xylem Sap Ph Increase: a Drought Signal Received at The
Plant Physiol. (1 997) 113: 559-573 Xylem Sap pH Increase: A Drought Signal Received at the Apoplastic Face of the Guard Cell That lnvolves the Suppression of Saturable Abscisic Acid Uptake by the Epidermal Symplast' Sally Wilkinson and William J. Davies* Division of Biological Sciences, lnstitute of Environmental and Biological Sciences, Lancaster University, Bailrigg, Lancaster, LAI 4YQ, United Kingdom is synthesized in roots that are in contact with drying soil Drought increased the pH of Commelina communis xylem sap (Davies and Zhang, 1991). The concentration of ABA in the from 6.1 to 6.7. Conductances of transpiring leaves were 50% xylem sap of well-watered sunflower plants collected by lower in pH 7.0 than in pH 6.0 buffers, but bulk leaf abscisic acid using techniques that match the transpirational flow rate of (ABA) concentration and shoot water status were unaffected by pH. intact plants is between 1.0 and 15.0 nmol dm-3 (Schurr et Stomatal apertures of isolated abaxial epidermis incubated on sim- al., 1992). In water-stressed sunflower plants the concen- ple buffers increased with external pH, so in vivo this must be tration of ABA can reach 3.0 pmol dmP3. Applications of overridden by alternative pH effects. Reductions in leaf transpira- similar concentrations of synthetic ABA to the transpira- tion rate at pH 7.0 were dependent on the presence of 1OP8 mo1 tion stream of detached leaves with normal water poten- dm-3 ABA in the xylem stream. We inferred that at pH 7.0 leaf apoplastic ABA concentrations increased: pH did not affect distri- tials commonly reduce water loss by approximately 50%. -

An Analytical Microscopical Study on the Role of the Exodermis in Apoplastic Rb+(K+) Transport in Barley Roots
Plant and Soil 207: 209–218, 1999. 209 © 1999 Kluwer Academic Publishers. Printed in the Netherlands. An analytical microscopical study on the role of the exodermis in apoplastic RbC(KC) transport in barley roots M. Gierth∗, R. Stelzer and H. Lehmann Institut für Tierökologie und Zellbiologie, Tierärztliche Hochschule Hannover, Bünteweg 17d, D-30559 Hannover, Germany Received: 29 June 1998. Accepted in revised form: 7 December 1998 Key words: Cryosectioning, endodermis, ion localisation, ion transport, rhizodermis, X-ray microanalysis Abstract The paper investigates how the apoplastic route of ion transfer is affected by the outermost cortex cell layers of a primary root. Staining of hand-made cross sections with aniline blue in combination with berberine sulfate demon- strated the presence of casparian bands in the endo- and exodermis, potentially being responsible for hindering apoplastic ion movement. The use of the apoplastic dye Evan’s Blue allowed viewing under a light microscope of potential sites of uncontrolled solute entry into the apoplast of the root cortex which mainly consisted of injured rhizodermis and/or exodermis cells. The distribution of the dye after staining was highly comparable to EDX analyses on freeze-dried cryosectioned roots. Here, we used RbC as a tracer for KC in a short-time application on selected regions of intact roots from intact plants. After subsequent quench-freezing with liquid propane the distribution of KC and RbC in cell walls was detected on freeze-dried cryosections by their specific X-rays resulting from the incident electrons in a SEM. All such attempts led to a single conclusion, namely, that the walls of the two outermost living cell sheaths of the cortex largely restrict passive solute movements into the apoplast. -

Apoplastic Route Cell Wall Symplastic Route Transmembrane Route Cytosol Key Plasmodesma Plasma Membrane Apoplast Symplast
CO O2 2 Light Sugar H2O O2 H2O and CO2 minerals © 2014 Pearson Education, Inc. 1 © 2014 Pearson Education, Inc. 2 Cell wall 24 32 42 29 40 16 Apoplastic route 11 19 21 27 34 8 3 6 Cytosol 14 13 Symplastic route 26 Shoot 1 5 apical 22 Transmembrane route meristem 9 Buds 18 10 4 31 2 17 23 7 12 Key 15 Plasmodesma 20 25 28 Plasma membrane Apoplast 1 mm Symplast © 2014 Pearson Education, Inc. 3 © 2014 Pearson Education, Inc. 4 CYTOPLASM EXTRACELLULAR + S H+ H FLUID H+ + H+ H + Hydrogen + H ion H H+ + S S H + H + Initial flaccid cell: + + H H H + ψP = 0 H+ H ψS = −0.7 H+ + S S S 0.4 M sucrose Proton H H+ ψ = −0.7 MPa Pure water: + solution: pump H ψP = 0 + ψP = 0 H /sucrose Sucrose Plasmolyzed ψS = 0 Turgid cell ψ = −0.9 (a) H+ and membrane potential cotransporter (neutral solute) cell at osmotic S ψ = 0 MPa at osmotic equilibrium ψ = −0.9 MPa equilibrium (b) H+ and cotransport of neutral solutes with its with its + + surroundings surroundings H − H 3 ψP = 0 ψP = 0.7 NO − + 3 H NO + ψS = −0.9 ψS = −0.7 + H+ K Potassium ion H ψ = −0.9 MPa ψ = 0 MPa + + H Nitrate K H+ K+ + − H+ K NO3 (a) Initial conditions: (b) Initial conditions: − + 3 NO − K cellular ψ > environmental ψ cellular ψ < environmental ψ 3 − NO + + NO3 K K H+ + − + H /NO3 + H cotransporter H Ion channel (c) H+ and cotransport of ions (d) Ion channels © 2014 Pearson Education, Inc. -

Topic: Absorption of Water B.Sc. Botany ( Sub.) II Group: C Dr
1 Topic: Absorption of Water B.Sc. Botany ( Sub.) II Group: C Dr. Sanjeev Kumar Vidyarthi Department of Botany Dr. L.K.V.D. College, Tajpur Absorption of Water Water is the most important plant nutrient and constitutes the greater part of the plant weight. Plants have the potentiality to absorb water through their entire surface right from root, stem, leaves, flowers, etc. However, as water is available mostly in the soil, only the underground root system is specialized to absorb water. Roots are often extensive and grow rapidly in the soil. In roots, the most efficient region of water absorption is the root hair zone. Each root hair zone has thousands of root hairs. Root hairs are specialized for water absorption. They are tubular outgrowths of 50-1500 μm (0.05-1.5 mm) length and 10 nm in breadth. Each root hair has a central vacuole filled with osmotically active cell sap and a peripheral cytoplasm. The wall is thin and permeable with pectic substances in the outer layer and cellulose on the inner layer. Root hairs pass into capillary micropores, get cemented to soil particles by pectic compounds and absorb capillary water. Pathways of Water Movements in Roots There are two pathways of water passage from root hairs to xylem inside the root- i. Apoplast ii. Symplast. Dr. Sanjeev Kumar Vidyarthi, Dept. of Botany, Dr. L.K.V.D. College, Tajpur 2 i. Apoplast Pathway Water passes from root hair to xylem through the walls of intervening cells without crossing any membrane or cytoplasm. The pathway provides the least resistance to movement of water. -

Ch. 36 Transport in Vascular Plants
Ch. 36 Transport in Vascular Plants Feb 41:32 PM 1 Essential Question: How does a tall tree get the water from its roots to the top of the tree? Feb 41:38 PM 2 Shoot architecture and Light Capture: Phyllotaxy arrangement of leaves on a stem to maximize light capture, reduce self shading determined by shoot apical meristem and specific to each species alternate = one leaf per node opposite = two leaves per node whorled = more than two leaves per node Norway spruce 1 is youngest leaf Apr 147:00 AM 3 leaf area index = ratio of total upper leaf surface of a single plant divided by surface area of land, normal value ~ 7 if above 7 leaves,branches undergo self pruning programmed cell death Mar 282:46 PM 4 Leaf orientations: horizontal leaf orientation for lowlight, capture sunlight more effectively vertical leaf orientation for high light, grasses, light rays coming in parallel to leaf so not too much light Apr 147:05 AM 5 Root architecture: mychorrhizae mutualistic relationship between fungi and roots 80% of land plants have this increases surface area for water and mineral absorption Mar 282:49 PM 6 Overview of transport in trees Feb 69:37 AM 7 Three types of transport in vascular plants: 1. transport of water and solutes by individual cells a. passive transport (osmosis) through aquaporins transport proteins water potential combined effects of solute concentration and physical pressure (esp. in plants due to cell wall) determines direction of movement of water free water moves high to low [ ] measured in megapascals (MPa) water potential = "0" in an open container (at sea level and rm. -

Vacuolar Symplast As a Regulated Pathway for Water Flows in Plants G
Russian Journal of Plant Physiology, Vol. 52, No. 3, 2005, pp. 326–331. Translated from Fiziologiya Rastenii, Vol. 52, No. 3, 2005, pp. 372–377. Original Russian Text Copyright © 2005 by Velikanov, Volobueva, Belova, Gaponenko. Vacuolar Symplast as a Regulated Pathway for Water Flows in Plants G. A. Velikanov, O. V. Volobueva, L. P. Belova, and E. M. Gaponenko Kazan Institute of Biochemistry and Biophysics, Kazan Research Center, Russian Academy of Sciences, a/ya 30, ul. Lobachevskogo 2/31, Kazan, Tatarstan, 420111 Russia; fax: 7 (8432) 38-7577; e-mail: [email protected] Received August 6, 2004 Abstract—Indirect immunofluorescent microscopy and a tonoplast-specific marker enzyme were used to dem- onstrate the occurrence of pyrophosphatase within the plasmodesmata in the elongation zone of maize root seg- ments. The pulsed field gradient NMR method (PFG NMR) was applied to study restricted self-diffusion of water molecules in the root segments under normal conditions and after the inhibition of respiration with sodium azide (10 mM NaN3, 30 min). The results led to the conclusion that vacuoles in the root segments exam- ined are interconnected into a unified intercellular continuum and that intervacuolar connections are formed by desmotubules within the plasmodesmata. The water permeability of the vacuolar symplast appears to be con- trolled by an ATP-dependent process. The experimental data can provide a methodological approach to study- ing water permeability of the vacuolar symplast with the PFG NMR technique. Key words: Zea mays - roots - plasmodesmata - desmotubules - vacuolar symplast - NMR INTRODUCTION spaces into a supracellular continuum. This means that vacuoles of neighboring cells are also interconnected. -
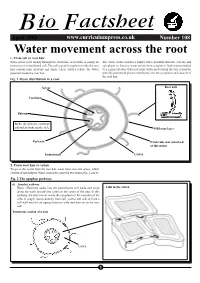
Water Movement Across the Root 1
BFio actsheet April 2002www.curriculumpress.co.uk Number108 Water movement across the root 1. From soil to root hair Water enters roots mainly through the root hairs. Aroot hair is simply an The water in the soil has a higher water potential than the cell sap and extension of an epidermal cell. The cell sap and cytoplasm inside the root cytoplasm so, because water moves from a region of high water potential hair contain ions, proteins and sugar. These solutes reduce the water to a region of lower water potential, water moves from the soil, across the potential inside the root hair. partially permeable plasma membrane, into the cytoplasm and vacuole of the root hair. Fig. 1 Tissue distribution in a root. Xylem Root hair Cambium Phloem Stele: the phloem, cambium and xylem make up the stele. Piliferous layer Pericycle Exodermis (not suberised at this stage) Endodermis Cortex 2. From root hair to xylem To get to the xylem from the root hair, water must cross the cortex, which consists of parenchyma. Water crosses the cortex by two routes (Fig. 2 and 3): Fig. 2 The apoplast pathway. (i) Apoplast pathway Water effectively soaks into the parenchyma cell walls and seeps Cells in the cortex along the walls towards the xylem in the centre of the root. In this pathway, the water never enters the cytoplasm or the vacuoles of the cells. It simply moves directly from cell wall to cell wall or from a cell wall into the air spaces between cells and then on to the next cell.