Radiological Significance of Thorium Processing in Manufacturing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Color 2012-13 Aug Newsletter.Pub
The Wildcat North Union School District’s Mission is to promote excellence and to prepare each student for success in the 21st Century. Welcome Back North Union It’s Time to……. “ACT LIKE A CHAMPION” The District enters the 2012-2013 school year with our best Report Card in history. North Union is projected to be rated “Excellent” by the Ohio Department of Education for the fifth year in a row. This year our theme is “Act Like A Champion”. We are no longer the little district hoping to achieve, but are building a tradition of championship performance in all that we do. It takes a greater dedication and work ethic to maintain and grow, than the journey to get here. When the final report comes out in August, we might have a chance at “Excellent with Distinction”. All buildings will be rated Excellent and have a Performance Index Score above 100. The district earned its sixth straight year of improvement on the Performance Index Score at 101.4. This data shows more North Union students are passing state tests in the accelerated and advanced ranges. The district has shown consistent growth in our Board Goals of passing all indicators at 85%, passing Adequate Yearly Progress and increasing our rate of accelerated and advanced scores. To review more data, please visit the NU website at www.n-union. k12.oh.us and click on “Annual Report”. Our big change this year will be the conversion to single route bussing. By August 10th, more details and a video for the drop -off loop at the elementary and middle schools will be posted on our website. -

Stations Monitored
Stations Monitored 10/01/2019 Format Call Letters Market Station Name Adult Contemporary WHBC-FM AKRON, OH MIX 94.1 Adult Contemporary WKDD-FM AKRON, OH 98.1 WKDD Adult Contemporary WRVE-FM ALBANY-SCHENECTADY-TROY, NY 99.5 THE RIVER Adult Contemporary WYJB-FM ALBANY-SCHENECTADY-TROY, NY B95.5 Adult Contemporary KDRF-FM ALBUQUERQUE, NM 103.3 eD FM Adult Contemporary KMGA-FM ALBUQUERQUE, NM 99.5 MAGIC FM Adult Contemporary KPEK-FM ALBUQUERQUE, NM 100.3 THE PEAK Adult Contemporary WLEV-FM ALLENTOWN-BETHLEHEM, PA 100.7 WLEV Adult Contemporary KMVN-FM ANCHORAGE, AK MOViN 105.7 Adult Contemporary KMXS-FM ANCHORAGE, AK MIX 103.1 Adult Contemporary WOXL-FS ASHEVILLE, NC MIX 96.5 Adult Contemporary WSB-FM ATLANTA, GA B98.5 Adult Contemporary WSTR-FM ATLANTA, GA STAR 94.1 Adult Contemporary WFPG-FM ATLANTIC CITY-CAPE MAY, NJ LITE ROCK 96.9 Adult Contemporary WSJO-FM ATLANTIC CITY-CAPE MAY, NJ SOJO 104.9 Adult Contemporary KAMX-FM AUSTIN, TX MIX 94.7 Adult Contemporary KBPA-FM AUSTIN, TX 103.5 BOB FM Adult Contemporary KKMJ-FM AUSTIN, TX MAJIC 95.5 Adult Contemporary WLIF-FM BALTIMORE, MD TODAY'S 101.9 Adult Contemporary WQSR-FM BALTIMORE, MD 102.7 JACK FM Adult Contemporary WWMX-FM BALTIMORE, MD MIX 106.5 Adult Contemporary KRVE-FM BATON ROUGE, LA 96.1 THE RIVER Adult Contemporary WMJY-FS BILOXI-GULFPORT-PASCAGOULA, MS MAGIC 93.7 Adult Contemporary WMJJ-FM BIRMINGHAM, AL MAGIC 96 Adult Contemporary KCIX-FM BOISE, ID MIX 106 Adult Contemporary KXLT-FM BOISE, ID LITE 107.9 Adult Contemporary WMJX-FM BOSTON, MA MAGIC 106.7 Adult Contemporary WWBX-FM -

Thermodynamic Properties of Thorium Dioxide from 298 to 1200 Ok
JO URNAL OF RESEARCH of the National Bureau of Standards-A. Physics and Chemistry Vol. 65, No.2, March- April 1961 Thermodynamic Properties of Thorium Dioxide From 298 to 1,200 oK Andrew C. Victor and Thomas B. Douglas (November 26, 1960) As a step in developing new standards of he.at ca l ~ac i ty applicable up to very h~gh temperatures, t he heat content (enthalpy) of thol'lum dlOxldoe, I h02 , relatn:,e to 2~3 K , was accurately measured at ten temperatures from 323 to 1,173 K . A Bunsen 1.ce ca l ol'l~neter and a drop method were used to make t he mea ~ urements on two samples of ":ldely d Iff erent bulk densities. The corresponding heat-capacity values for the hIgher denSIty sample ~re represented within t heir uncertain ty (estimated to be ± 0.3 to 0.5 %) by the followlI1g empirical equation 1 (cal mole- I deg- I at T OK) : C ~ = 17 . 057 + 1 8. 06 ( 10 -4) T - 2.5166 (1 05)/1'2 At 298 oK t his equation agrees with previously reported low-temperatu.re measurements made with an adiabatic calorimeter. Values of heat content, heat capaelty, entropy, and Gibb's free energy function are tabulated from 298.15 t o 1,200 oK. 1. Introduction measll remen ts arc soon to be extended up to ap proximately 1,800 OK. Current practical and theoretical developm~l!ts However, at higher temperatures aluminum oxide have increased the need for accurate heat capaCltlOs is impractical as a heat standard, for it becomes and related thermal properties at high temp ~rature s, increasingly volatile, and melts at approximately yet the values reported for the same mat~nal from 2,300 OK. -

Extraction of Thorium Oxide from Monazite Ore
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Supervised Undergraduate Student Research Chancellor’s Honors Program Projects and Creative Work 5-2019 Extraction of Thorium Oxide from Monazite Ore Makalee Ruch University of Tennessee, Knoxville, [email protected] Chloe Frame University of Tennessee, Knoxville Molly Landon University of Tennessee, Knoxville Ralph Laurel University of Tennessee, Knoxville Annabelle Large University of Tennessee, Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_chanhonoproj Part of the Environmental Chemistry Commons, Geological Engineering Commons, and the Other Chemical Engineering Commons Recommended Citation Ruch, Makalee; Frame, Chloe; Landon, Molly; Laurel, Ralph; and Large, Annabelle, "Extraction of Thorium Oxide from Monazite Ore" (2019). Chancellor’s Honors Program Projects. https://trace.tennessee.edu/utk_chanhonoproj/2294 This Dissertation/Thesis is brought to you for free and open access by the Supervised Undergraduate Student Research and Creative Work at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Chancellor’s Honors Program Projects by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Extraction of Thorium Oxide from Monazite Ore Dr. Robert Counce Department of Chemical and Biomolecular Engineering University of Tennessee Chloe Frame Molly Landon Annabel Large Ralph Laurel Makalee Ruch CBE 488: Honors -

Fabrication of Thorium and Thorium Dioxide
Natural Science, 2015, 7, 10-17 Published Online January 2015 in SciRes. http://www.scirp.org/journal/ns http://dx.doi.org/10.4236/ns.2015.71002 Fabrication of Thorium and Thorium Dioxide Balakrishna Palanki (Retired) Nuclear Fuel Complex, Hyderabad, India Email: [email protected] Received 10 November 2014; revised 9 December 2014; accepted 28 December 2014 Copyright © 2015 by author and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Thorium based nuclear fuel is of immense interest to India by virtue of the abundance of Thorium and relative shortage of Uranium. Thorium metal tubes were being cold drawn using copper as cladding to prevent die seizure. After cold drawing, the copper was removed by dissolution in ni- tric acid. Thorium does not dissolve being passivated by nitric acid. Initially the copper cladding was carried out by inserting copper tubes inside and outside the thorium metal tube. In an inno- vative development, the mechanical cladding with copper was replaced by electroplated copper with a remarkable improvement in thorium tube acceptance rates. Oxalate derived thoria powder was found to require lower compaction pressures compared to ammonium diuranate derived urania powders to attain the same green compact density. However, the green pellets of thoria were fragile and chipped during handling. The strength improved after introducing a ball milling step before compaction and maintaining the green density above the specified value. Alternatively, binders were used later for greater handling strength. Magnesia was conventionally being used as dopant to enhance the sinterability of thoria. -

Aec-Nasa Tech Brief
March 1969 Brief 69-10079 sty C AEC-NASA TECH BRIEF YES AEC-NASA Tech Briefs describe innovations resulting from the research and development program of the U.S. AEC or from AEC-NASA interagency efforts. They are issued to encourage commercial application. Tech Briefs are published by NASA and may be purchased, at 15 cents each, from the Clearinghouse for Federal Scientific and Technical Information, Springfield, Virginia 22151. Preparation of Thorium by Magnesium—Zinc Reduction Stirring Motor Ball Valve Stuffing Box Assembly Sampling Port - Water Annulus Graphitar Bearing 1___._Hea t Shields SST Shaft Ta Thermowell Graphite Secondary Crucible Heated Ta Shaft Graphite Pouring Mold Baffled Crucible^v I SST Shell Ta Paddle THORIUM DIOXIDE REDUCTION APPARATUS A technique for the preparation of thorium metal success of the process. by magnesium—zinc reduction of thorium dioxide has The present method for commercial production of been investigated on a laboratory scale. The extent thorium metal involves metallothermic reduction of of reduction was determined as a function of time, thorium tetrafluoride with calcium in the presence of amount and composition of flux, temperature, agita- zinc chloride. However, calcium is expensive as a re- tion, particle size of the oxide, and magnesium con- ductant and the process requires a prior hydro-fluo- centration in the alloy. It was found that the use of rination step. Using magnesium—zinc alloy for the a flux of the proper composition is essential to the reduction of thorium dioxide has potential economic (continued overleaf) This document was prepared under the sponsorship of the Atomic liability resulting from the use of the information contained in this Energy Commission and/or the National Aeronautics and Space document, or warrants that the use of any information, apparatus, Administration. -
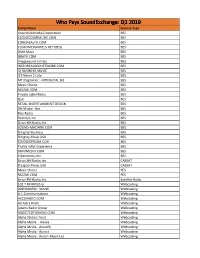
Licensee Count Q1 2019.Xlsx
Who Pays SoundExchange: Q1 2019 Entity Name License Type Aura Multimedia Corporation BES CLOUDCOVERMUSIC.COM BES COROHEALTH.COM BES CUSTOMCHANNELS.NET (BES) BES DMX Music BES GRAYV.COM BES Imagesound Limited BES INSTOREAUDIONETWORK.COM BES IO BUSINESS MUSIC BES It'S Never 2 Late BES MTI Digital Inc - MTIDIGITAL.BIZ BES Music Choice BES MUZAK.COM BES Private Label Radio BES Qsic BES RETAIL ENTERTAINMENT DESIGN BES Rfc Media - Bes BES Rise Radio BES Rockbot, Inc. BES Sirius XM Radio, Inc BES SOUND-MACHINE.COM BES Stingray Business BES Stingray Music USA BES STUDIOSTREAM.COM BES Thales Inflyt Experience BES UMIXMEDIA.COM BES Vibenomics, Inc. BES Sirius XM Radio, Inc CABSAT Stingray Music USA CABSAT Music Choice PES MUZAK.COM PES Sirius XM Radio, Inc Satellite Radio 102.7 FM KPGZ-lp Webcasting 999HANKFM - WANK Webcasting A-1 Communications Webcasting ACCURADIO.COM Webcasting Ad Astra Radio Webcasting Adams Radio Group Webcasting ADDICTEDTORADIO.COM Webcasting Aloha Station Trust Webcasting Alpha Media - Alaska Webcasting Alpha Media - Amarillo Webcasting Alpha Media - Aurora Webcasting Alpha Media - Austin-Albert Lea Webcasting Alpha Media - Bakersfield Webcasting Alpha Media - Biloxi - Gulfport, MS Webcasting Alpha Media - Brookings Webcasting Alpha Media - Cameron - Bethany Webcasting Alpha Media - Canton Webcasting Alpha Media - Columbia, SC Webcasting Alpha Media - Columbus Webcasting Alpha Media - Dayton, Oh Webcasting Alpha Media - East Texas Webcasting Alpha Media - Fairfield Webcasting Alpha Media - Far East Bay Webcasting Alpha Media -

Chemical Compatibility Storage Group
CHEMICAL SEGREGATION Chemicals are to be segregated into 11 different categories depending on the compatibility of that chemical with other chemicals The Storage Groups are as follows: Group A – Compatible Organic Acids Group B – Compatible Pyrophoric & Water Reactive Materials Group C – Compatible Inorganic Bases Group D – Compatible Organic Acids Group E – Compatible Oxidizers including Peroxides Group F– Compatible Inorganic Acids not including Oxidizers or Combustible Group G – Not Intrinsically Reactive or Flammable or Combustible Group J* – Poison Compressed Gases Group K* – Compatible Explosive or other highly Unstable Material Group L – Non-Reactive Flammable and Combustible, including solvents Group X* – Incompatible with ALL other storage groups The following is a list of chemicals and their compatibility storage codes. This is not a complete list of chemicals, but is provided to give examples of each storage group: Storage Group A 94‐75‐7 2,4‐D (2,4‐Dichlorophenoxyacetic acid) 94‐82‐6 2,4‐DB 609-99-4 3,5-Dinitrosalicylic acid 64‐19‐7 Acetic acid (Flammable liquid @ 102°F avoid alcohols, Amines, ox agents see SDS) 631-61-8 Acetic acid, Ammonium salt (Ammonium acetate) 108-24-7 Acetic anhydride (Flammable liquid @102°F avoid alcohols see SDS) 79‐10‐7 Acrylic acid Peroxide Former 65‐85‐0 Benzoic acid 98‐07‐7 Benzotrichloride 98‐88‐4 Benzoyl chloride 107-92-6 Butyric Acid 115‐28‐6 Chlorendic acid 79‐11‐8 Chloroacetic acid 627‐11‐2 Chloroethyl chloroformate 77‐92‐9 Citric acid 5949-29-1 Citric acid monohydrate 57-00-1 Creatine 20624-25-3 -

530 CIAO BRAMPTON on ETHNIC AM 530 N43 35 20 W079 52 54 09-Feb
frequency callsign city format identification slogan latitude longitude last change in listing kHz d m s d m s (yy-mmm) 530 CIAO BRAMPTON ON ETHNIC AM 530 N43 35 20 W079 52 54 09-Feb 540 CBKO COAL HARBOUR BC VARIETY CBC RADIO ONE N50 36 4 W127 34 23 09-May 540 CBXQ # UCLUELET BC VARIETY CBC RADIO ONE N48 56 44 W125 33 7 16-Oct 540 CBYW WELLS BC VARIETY CBC RADIO ONE N53 6 25 W121 32 46 09-May 540 CBT GRAND FALLS NL VARIETY CBC RADIO ONE N48 57 3 W055 37 34 00-Jul 540 CBMM # SENNETERRE QC VARIETY CBC RADIO ONE N48 22 42 W077 13 28 18-Feb 540 CBK REGINA SK VARIETY CBC RADIO ONE N51 40 48 W105 26 49 00-Jul 540 WASG DAPHNE AL BLK GSPL/RELIGION N30 44 44 W088 5 40 17-Sep 540 KRXA CARMEL VALLEY CA SPANISH RELIGION EL SEMBRADOR RADIO N36 39 36 W121 32 29 14-Aug 540 KVIP REDDING CA RELIGION SRN VERY INSPIRING N40 37 25 W122 16 49 09-Dec 540 WFLF PINE HILLS FL TALK FOX NEWSRADIO 93.1 N28 22 52 W081 47 31 18-Oct 540 WDAK COLUMBUS GA NEWS/TALK FOX NEWSRADIO 540 N32 25 58 W084 57 2 13-Dec 540 KWMT FORT DODGE IA C&W FOX TRUE COUNTRY N42 29 45 W094 12 27 13-Dec 540 KMLB MONROE LA NEWS/TALK/SPORTS ABC NEWSTALK 105.7&540 N32 32 36 W092 10 45 19-Jan 540 WGOP POCOMOKE CITY MD EZL/OLDIES N38 3 11 W075 34 11 18-Oct 540 WXYG SAUK RAPIDS MN CLASSIC ROCK THE GOAT N45 36 18 W094 8 21 17-May 540 KNMX LAS VEGAS NM SPANISH VARIETY NBC K NEW MEXICO N35 34 25 W105 10 17 13-Nov 540 WBWD ISLIP NY SOUTH ASIAN BOLLY 540 N40 45 4 W073 12 52 18-Dec 540 WRGC SYLVA NC VARIETY NBC THE RIVER N35 23 35 W083 11 38 18-Jun 540 WETC # WENDELL-ZEBULON NC RELIGION EWTN DEVINE MERCY R. -

A Computational Analysis of Thorium Dioxide and Th(1-X)Uxo2 Systems
University College London A Computational Analysis of Thorium Dioxide and Th(1-x)UxO2 Systems Thesis submitted for the degree of Doctor of Philosophy (PhD) by Ashley Elizabeth Shields Supervisor: Professor Nora H. de Leeuw University College London Department of Chemistry December 2015 Declaration I, Ashley Elizabeth Shields, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been properly indicated and fully acknowledged in the thesis. Ashley Elizabeth Shields December 2015 2 Abstract Nuclear power generation is an important way to satisfy rising global energy needs without increasing dependence on coal and petroleum. However, conventional nuclear fuels, such as uranium and plutonium dioxides, raise several safety concerns. Many countries have shown a renewed interest in thorium-based fuels as a potentially safer alternative. Thorium dioxide requires small amounts of a neutron source, such as uranium or plutonium, to generate a sustainable fission reaction. Due to the hazards of conducting experimental work on radioactive substances, a robust theoretical understanding of this doped- ThO2 fuel is needed. Using Density Functional Theory (DFT), we have studied the effects of uranium addition on the electronic structures of both the pure thoria bulk structure and three flat surfaces of ThO2 and simulated Scanning Tunneling Microscopy (STM) images of each surface. We have also studied the effect of a uranium adatom on these surfaces. However, we wished to study larger systems than are practical to simulate with DFT, and so we developed a new Th-O Buckingham- type force field that has been optimized to work with a leading UO2 interatomic potential. -

Exhibit 2181
Exhibit 2181 Case 1:18-cv-04420-LLS Document 131 Filed 03/23/20 Page 1 of 4 Electronically Filed Docket: 19-CRB-0005-WR (2021-2025) Filing Date: 08/24/2020 10:54:36 AM EDT NAB Trial Ex. 2181.1 Exhibit 2181 Case 1:18-cv-04420-LLS Document 131 Filed 03/23/20 Page 2 of 4 NAB Trial Ex. 2181.2 Exhibit 2181 Case 1:18-cv-04420-LLS Document 131 Filed 03/23/20 Page 3 of 4 NAB Trial Ex. 2181.3 Exhibit 2181 Case 1:18-cv-04420-LLS Document 131 Filed 03/23/20 Page 4 of 4 NAB Trial Ex. 2181.4 Exhibit 2181 Case 1:18-cv-04420-LLS Document 132 Filed 03/23/20 Page 1 of 1 NAB Trial Ex. 2181.5 Exhibit 2181 Case 1:18-cv-04420-LLS Document 133 Filed 04/15/20 Page 1 of 4 ATARA MILLER Partner 55 Hudson Yards | New York, NY 10001-2163 T: 212.530.5421 [email protected] | milbank.com April 15, 2020 VIA ECF Honorable Louis L. Stanton Daniel Patrick Moynihan United States Courthouse 500 Pearl St. New York, NY 10007-1312 Re: Radio Music License Comm., Inc. v. Broad. Music, Inc., 18 Civ. 4420 (LLS) Dear Judge Stanton: We write on behalf of Respondent Broadcast Music, Inc. (“BMI”) to update the Court on the status of BMI’s efforts to implement its agreement with the Radio Music License Committee, Inc. (“RMLC”) and to request that the Court unseal the Exhibits attached to the Order (see Dkt. -

Thermodynamic Model for the Solubility of Thorium Dioxide in The
Radiochim. Acta 88, 2972306 (2000) by Oldenbourg Wissenschaftsverlag, München Thermodynamic model for the solubility of thorium dioxide + – – ° ° in the Na -Cl -OH -H2O system at 23 C and 90 C By Dhanpat Rai1,*, Dean A. Moore1, Charles S. Oakes1 and Mikazu Yui2 1 Pacific Northwest National Laboratory, Richland, Washington 99352, USA 2 Japan Nuclear Cycle Development Institute, Tokai Works, Tokai, Japan (Received August 30, 1999; accepted in revised form March 8, 2000) Solubility / Thermodynamics / Thorium dioxide / ThO2(c) / at room temperature. Therefore, potential thorium concen- ThO2(am) / Temperature effects on ThO2 crystallinity / trations in aqueous solutions contacting failed repositories Solubility product / Solubility products at different may be many orders of magnitude lower than indicated by temperatures measurements of ThO2(am) solubility at 25 °C. At 25 °C, the precipitation/dissolution kinetics of Summary. Data are extremely limited on the effects of tem- ThO2(c) are not known, which makes it difficult to accu- perature on crystallinity and the resulting changes in solubility rately ascertain the solubility product of ThO2(c) at this products of thermally transformed thorium oxide phases. Such temperature. Fortunately, in addition to being relevant to data are required to reliably predict thorium behavior in high- expected repository conditions, a moderate increase in tem- level waste repositories where higher than ambient tempera- perature accelerates the precipitation/dissolution kinetics tures are expected. Solubility studies were conducted as a func- such that the solubility of ThO2(c) can be approached from tion of pH and time and at 0.1 M NaCl for 1) ThO2(am) at both the oversaturation and undersaturation directions.