Toxicological Profile for Thorium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
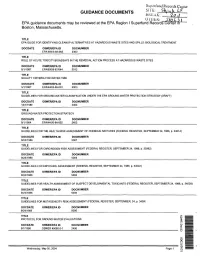
List of Guidance Documents for Shpack Landfill Record
Super fund Records Center GUIDANCE DOCUMENTS bKiS.K- ' 2 "7 OHihR: 2tofe *, EPA guidance documents may be reviewed at the EPA Region I Superfund Records Center inl Boston, Massachusetts. TITLE EPA GUIDE FOR IDENTIFYING CLEANUP ALTERNATIVES AT HAZARDOUS-WASTE SITES AND SPILLS: BIOLOGICAL TREATMENT DOCDATE OSWER/EPA ID DOCNUMBER EPA-600/3-83-063 2303 TITLE ROLE OF ACUTE TOXICITY BIOASSAYS IN THE REMEDIAL ACTION PROCESS AT HAZARDOUS WASTE SITES DOCDATE OSWER/EPA ID DOCNUMBER 8/1/1987 EPA/600/8-87/044 5012 TITLE QUALITY CRITERIA FOR WATER 1986 DOCDATE OSWER/EPA ID DOCNUMBER 5/1/1987 EPA/440/5-86-001 4003 TITLE GUIDELINES FOR GROUND-WATER CLASSIFICATION UNDER THE EPA GROUND-WATER PROTECTION STRATEGY (DRAFT) DOCDATE OSWER/EPA ID DOCNUMBER 12/1/1986 2404 TITLE GROUND-WATER PROTECTION STRATEGY DOCDATE OSWER/EPA ID DOCNUMBER 8/1/1984 EPA/440/6-84-002 2403 TITLE GUIDELINES FOR THE HEALTH RISK ASSESSMENT OF CHEMICAL MIXTURES (FEDERAL REGISTER, SEPTEMBER 24, 1986, p. 34014) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5007 TITLE GUIDELINES FOR CARCINOGEN RISK ASSESSMENT (FEDERAL REGISTER, SEPTEMBER 24, 1986, p. 33992) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5003 TITLE GUIDELINES FOR EXPOSURE ASSESSMENT (FEDERAL REGISTER, SEPTEMBER 24, 1986, p. 34042) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5004 TITLE GUIDELINES FOR HEALTH ASSESSMENT OF SUSPECT DEVELOPMENTAL TOXICANTS (FEDERAL REGISTER, SEPTEMBER 24,1986, p. 34028) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5005 TITLE GUIDELINES FOR MUTAGENICITY RISK ASSESSMENT (FEDERAL REGISTER, SEPTEMBER, 24, p. 34006 DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5006 TITLE PROTOCOL FOR GROUND-WATER EVALUATIONS DOCDATE OSWER/EPA ID DOCNUMBER 9/1/1986 OSWER #9080.0-1 2406 Wednesday, May 05, 2004 Page 1 GUIDANCE DOCUMENTS EPA guidance documents may be reviewed at the EPA Region I Superfund Records Center in Boston, Massachusetts. -
Thallium-201 for Medical Use. I
THALLIUM-201 FOR MEDICAL USE. I E. Lebowitz, M. W. Greene, R. Fairchild, P. R. Bradley-Moore, H. 1. Atkins,A. N. Ansari, P. Richards,and E. Belgrave Brookhaven National Laboratory Thallium-201 merits evaluation for myocar tumors (7—9), the use of radiothallium should also dial visualization, kidney studies, and tumor be evaluated for this application. diagnosis because of its physical and biologic Thallium-201 decays by electron capture with a properties. A method is described for prepara 73-hr half-life. It emits mercury K-x-rays of 69—83 tion of this radiopharmaceutical for human use. keY in 98% abundance plus gamma rays of I 35 and A critical evaluation of 501T1 and other radio 167 keV in 10% total abundance. Because of its pharmaceuticals for myocardial visualization is good shelf-life, photon energies, and mode of decay, given. 201T1was the radioisotope of thallium chosen for development. Thallium-20 1 is a potentially useful radioisotope MATERIALS AND METHODS for various medical applications including myocardial Thallium-201 is produced by irradiating a natural visualization and possible assessment of physiology, thallium target in the external beam of the 60-in. as a renal medullary imaging agent, and for tumor Brookhaven cyclotron with 3 1-MeV protons. The detection. nuclear reaction is 203Tl(p,3n)201Pb. Lead-201 has The use of radiothallium in nuclear medicine was a half-life of 9.4 hr and is the parent of 201T1.The first suggested by Kawana, et al (1 ) . In terms of thallium target, fabricated from an ingot of 99.999% organ distribution (2) and neurophysiologic function pure natural thallium metal (29.5% isotopic abun (3), thallium is biologically similar to potassium. -

Corrosion of Refractories by Denis A
A Chapter in the Refractories Handbook, edited by Charles A. Schacht, and to be published by Marcel Dekker, Inc., New York, NY 10016 Corrosion of Refractories by Denis A. Brosnan, PhD, PE Clemson University Clemson, SC 29634-0971 Introduction Refractories are used at elevated temperatures for structural purposes and they are used in many cases to contain a high temperature corrosive environment. This corrosive environment usually contains liquid (melted) phases that participate in chemical reactions with the refractory at the elevated temperatures resulting in refractory consumption or wear. It is usually not immediately obvious, but the oxidation and reduction state of the environment (as “redox” conditions or oxygen “activity”) can participate in and influence the chemical reactions that take place. Along with chemical reactions during corrosion, physical changes occur that may be accelerated by the corrosion process. Corrosion of refractories can be defined for the purposes of this discussion as follows: Corrosion of Refractories – refractory wear by loss of thickness and mass from the exposed face of the refractory as a consequence of chemical attack by a corroding fluid in a process in which the refractory and the corroding fluid react approaching chemical equilibrium in the zone of contact between the refractory and the fluid. It is an essential point that corrosion reactions proceed in a direction toward localized chemical equilibrium. This means that phase equilibrium diagrams can be used to analyze corrosion situations and to predict chemical strategies to minimize corrosion and wear rates. This gives persons interested in refractory corrosion two options. The first is to view corrosion as a chemical and physical process without a detailed application of phase equilibrium diagrams – called the “phenomenological approach”. -

Th Thorium Compounds with S, Se, Te and B
springer.com Chemistry : Chemistry (general) Brown, David, Wedemeyer, Horst, Buschbeck, Karl-Christian, Keller, Cornelius (Editors-in-chief.) Th Thorium Compounds with S, Se, Te and B The present volume, Thorium C5, deals with the compounds of thorium and sulfur, selenium, tellurium, and boron, as well as with oxoacid compounds of the three chalcogen elements. Thorium borates have already been treated in Thorium C2. In contrast to the corresponding compounds of uranium the thorium sulfides, etc. , do not show any nuclear or other technological application; they are only of academic interest, despite some very interest• ing electronic properties, especially of the 1 : 1 compounds. The thorium-sulfur and the thorium• boron systems in particular were studied in detail, so that we have a clear picture of them, whereas there are still a lot of open questions in the systems Th-Se and Th-Te - not very different from other metal chalcogenide systems. Thorium sulfates are of some technological importance because they are formed in solution during recovery of thorium from monazite by sulfuric acid leaching. The very detailed and critical treatment of the chemical and physical Springer properties of the compounds discussed also enables us to find gaps still remaining in our 8th ed. 1985, XIX, 149 p. knowledge and thus to initiate new research in this field. I want to thank the two authors, Dr. 8th 28 illus., 1 illus. in color. edition Horst Wedemeyer (Karlsruhe) and Dr. David Brown (Harwell), for their excellent contributions, the "Literaturabteilung" of the Karlsruhe Nuclear Research Center for its help in providing reports and other documents difficult to procure, as well as the staff of the Gmelin-Institute, especially to Dr. -

Effect of Natural Organic Matter on Thallium and Silver Speciation Loïc Martin, Caroline Simonucci, Sétareh Rad, Marc F
Effect of natural organic matter on thallium and silver speciation Loïc Martin, Caroline Simonucci, Sétareh Rad, Marc F. Benedetti To cite this version: Loïc Martin, Caroline Simonucci, Sétareh Rad, Marc F. Benedetti. Effect of natural organic matter on thallium and silver speciation. Journal of Environmental Sciences, Elsevier, 2020, 93, pp.185-192. 10.1016/j.jes.2020.04.001. hal-02565435 HAL Id: hal-02565435 https://hal.archives-ouvertes.fr/hal-02565435 Submitted on 6 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Title Page (with Author Details) Effect of natural organic matter on thallium and silver speciation Loïc A. Martin1,2, Caroline Simonucci2, Sétareh Rad3, and Marc F. Benedetti1* 1Université de Paris, Institut de physique du Globe de Paris, CNRS, F-75005 Paris, France. 2IRSN, PSE-ENV/SIRSE/LER-Nord, BP 17, 92262 Fontenay-aux-Roses Cedex, France 3BRGM, Unité de Géomicrobiologie et Monitoring environnemental 45060 Orléans Cedex 2, France. *Corresponding authors. Email address: [email protected] Manuscript File Click here to access/download;Graphical Abstract;graphical abstract.001.jpeg Manuscript File Click here to view linked References 23 Effect of natural organic matter on thallium and silver speciation 24 25 Loïc A. -

Untraditional Synthesis of Boron-Containing Superhard and Refractory Materials - a Review B
G.J. E.D.T., Vol. 2(1) 2013:21-26 ISSN 2319 – 7293 UNTRADITIONAL SYNTHESIS OF BORON-CONTAINING SUPERHARD AND REFRACTORY MATERIALS - A REVIEW B. Agyei-Tuffour1,2, E. Annan1,2, E. R. Rwenyagila1, E. Ampaw1, E. Arthur1, K. Mustapha1, S. Kolawole1, W. O. Soboyejo1,3, & D. D. Radev1,4 1Department of Materials Science and Engineering, African University of Science and Technology, Abuja-Nigeria 2Department of Materials Science and Engineering, Private Mail Bag, University of Ghana, Legon-Accra. 3Department of Mechanical and Aerospace Engineering, Princeton University, USA. 4Institute of General and Inorganic Chemistry, Bulgaria Academy of Sciences, Sofia-Bulgaria Abstract Boron-containing ceramics find large application in production of superhard and high-temperature materials with application in nuclear and aerospace techniques, military industry etc. The synthesis methods are decisive for the complexity of chemical, morphological and technological properties of these materials. The traditional high-temperature synthesis methods have some disadvantages leading to inconstancy of the product composition due to the boron evaporation, degradation of the furnace materials and contamination of the products, high energy losses etc. Here we show the advantages of some untraditional synthesis methods like direct mechanical synthesis and self propagating high-temperature synthesis (SHS) in the production of titanium diboride (TiB2), zirconium diboride (ZrB2) and production of dense boron carbide (B4C) based materials. Using SEM, TEM, XRD and analytical chemical methods, it was shown that diborides of titanium and zirconium have appropriate properties for production of dense ceramic materials. Using the method of mechanically-assisted sintering high-dense B4C-based ceramic materials was obtained. It was shown that the mechanical properties of materials obtained by pressureless sintering are close or overcome the corresponding properties of boron carbide densified by the method of hot pressing. -

Study of Rare Earth Elements, Uranium and Thorium Migration in Rocks from Espinharas Uranium Deposit, Paraiba - Brazil
2009 International Nuclear Atlantic Conference - INAC 2009 Rio de Janeiro,RJ, Brazil, September27 to October 2, 2009 ASSOCIAÇÃO BRASILEIRA DE ENERGIA NUCLEAR - ABEN ISBN: 978-85-99141-03-8 STUDY OF RARE EARTH ELEMENTS, URANIUM AND THORIUM MIGRATION IN ROCKS FROM ESPINHARAS URANIUM DEPOSIT, PARAIBA - BRAZIL Cirilo C.S. Conceição Instituto de Radioproteção e Dosimetria -IRD Av. salvador Allend, S/N - Jacarepaguá Rio de Janeiro, Brazil CEP.: 22780-160 [email protected] ABSTRACT The determination of Rare Earth Elements as natural analogue in patterns geologic has grown as a tool for predicting the long-term safety of nuclear disposal in geological formation. Migration of natural radionuclides is one of the most serious problems in the waste deposit from nuclear fuel cycle. Rare Earth Elements show the same kinetic behavior in rocks as natural radionuclides. This similar property of the analogues allows perform studies and models on the subject of radionuclides migration. The aim of this study was to determine the distribution of Rare Earth Elements in rocks located at Espinharas – Paraíba – Brazil, uranium deposit. In this work are presented the results from the study above the distribution of rare earth elements in function of the degree of mineralized rocks, composition and the conditions of radioactive equilibrium of the uranium and thorium in some fractures on the rocks from radioactive occurrence of Espinharas-Brazil. The results show that there is a correlation of heavy Rare Earth Elements, uranium and Thorium concentrations to oxidation factor of the rocks. However this correlation was not observed for light Rare Earth Elements. It means that heavy Rare earth Elements follow the natural radionuclides in oxidation process of rocks. -

Environmental Health Criteria 126 Partially Halogenated Chlorofluorocarbons
Environmental Health Criteria 126 Partially halogenated chlorofluorocarbons ( methane derivatives) Please note that the layout and pagination of this web version are not identical with the printed version. Chlorofluorocarbons, partially halogenated (methane derivatives) (EHC 126, 1991) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 126 PARTIALLY HALOGENATED CHLOROFLUROCARBONS (METHANE DERIVATIVES) This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization Draft prepared by Professor D. Beritic-Stahuljak and Professor F. Valic, University of Zagreb, Yugoslavia, using texts made available by Dr. D.S. Mayer, Hoechst AG, Frankfurt am Main, Germany and by Dr. I.C. Peterson and Dr. G.D. Wade, ICI Central Toxicological Laboratory, Macclesfield, United Kingdom World Health Organization Geneva, 1991 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Measurements of Fission Cross Sections for the Isotopes Relevant to the Thorium Fuel Cycle
EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH CERN/INTC 2001-025 INTC/P-145 08 Aug 2001 Measurements of Fission Cross Sections for the Isotopes relevant to the Thorium Fuel Cycle The n_TOF Collaboration ABBONDANNO U.20, ANDRIAMONJE S.10, ANDRZEJEWSKI J.24, ANGELOPOULOS A.12, ASSIMAKOPOULOS P.15, BACRI C-O.8, BADUREK G.1, BAUMANN P.9, BEER H.11, BENLLIURE J.32, BERTHIER B.8, BONDARENKO I.26, BOS A.J.J.36, BUSTREO N.19, CALVINO F.33, CANO-OTT D.28, CAPOTE R.31, CARLSON P.34, CHARPAK G.5, CHAUVIN N.8, CENNINI P.5, CHEPEL V.25, COLONNA N.20, CORTES G.33, CORTINA D.32, CORVI F.23, DAMIANOGLOU D.16, DAVID S.8, DIMOVASILI E.12, DOMINGO C.29, DOROSHENKO A.27, DURAN ESCRIBANO I.32, ELEFTHERIADIS C.16, EMBID M.28, FERRANT L.8, FERRARI A.5, FERREIRA–MARQUES R.25, FRAIS–KOELBL H.3, FURMAN W.26, FURSOV B.27, GARZON J.A.32, GIOMATARIS I.10, GLEDENOV Y.26, GONZALEZ–ROMERO E.28, GOVERDOVSKI A.27, GRAMEGNA F.19, GRIESMAYER E.3, GUNSING F.10, HAIGHT R.37, HEIL M.11, HOLLANDER P.36, IOANNIDES K.G.15, IOANNOU P.12, ISAEV S.27, JERICHA E.1, KADI Y.5, KAEPPELER F.11, KARADIMOS D.17, KARAMANIS D.15, KAYUKOV A.26, KAZAKOV L.27, KELIC A.9, KETLEROV V.27, KITIS G.16, KOEHLER P.E.38., KOPACH Y.26, KOSSIONIDES E.14, KROSHKINA I.27, LACOSTE V.5, LAMBOUDIS C.16, LEEB H.1, LEPRETRE A.10, LOPES M.25, LOZANO M.31, MARRONE S.20, MARTINEZ-VAL J. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

BISMUTH LEAD ALLOY Synonyms BISMUTH ALLOY, 5805, HS CODE 78019900 ● NYRSTAR LEAD ALLOYS
1. IDENTIFICATION OF THE MATERIAL AND SUPPLIER 1.1 Product identifier Product name BISMUTH LEAD ALLOY Synonyms BISMUTH ALLOY, 5805, HS CODE 78019900 ● NYRSTAR LEAD ALLOYS 1.2 Uses and uses advised against Uses ALLOY Used for further refining of bismuth and lead metals. 1.3 Details of the supplier of the product Supplier name NYRSTAR PORT PIRIE Address PO Box 219, Port Pirie, SA, 5540, AUSTRALIA Telephone (08) 8638 1500 Fax (08) 8638 1583 Website http://www.nyrstar.com 1.4 Emergency telephone numbers Emergency (08) 8638 1500 2. HAZARDS IDENTIFICATION 2.1 Classification of the substance or mixture CLASSIFIED AS HAZARDOUS ACCORDING TO SAFE WORK AUSTRALIA CRITERIA GHS classifications Acute Toxicity: Oral: Category 4 Acute Toxicity: Inhalation: Category 4 Toxic to Reproduction: Category 1A Specific Target Organ Systemic Toxicity (Repeated Exposure): Category 2 2.2 GHS Label elements Signal word DANGER Pictograms Hazard statements H302 Harmful if swallowed. H332 Harmful if inhaled. H360 May damage fertility or the unborn child. H373 May cause damage to organs through prolonged or repeated exposure. Prevention statements P202 Do not handle until all safety precautions have been read and understood. P260 Do not breathe dust/fume/gas/mist/vapours/spray. P264 Wash thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P271 Use only outdoors or in a well-ventilated area. P281 Use personal protective equipment as required. SDS Date: 08 Jun 2018 Page 1 of 7 Version No: 1.3 PRODUCT NAME BISMUTH LEAD ALLOY Response statements P304 + P340 IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.