I: Consideration of Embryogenesis in the Normal Heart R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Truncus Arteriosus Communis: Report of Three Cases and Review of Literature
Truncus arteriosus communis: report of three cases and review of literature Henriette Poaty1,2, Fanny Pelluard3, Gwenaelle André3, Brigitte Maugey-Laulom4, Dominique Carles3 1. Histology-Embryology and Genetic Laboratory, Faculty of Health Sciences, BP 2672, Marien Ngouabi University, Brazzaville, Congo. 2. National Research Institute on Health Sciences, Brazzaville, Congo 3. Department of Fetopathology, CHU Pellegrin, place Amélie Raba, 33076 Bordeaux cedex France 4. Fetal Imaging Unit, Maternity, CHU Pellegrin, place Amélie Raba, 33076 Bordeaux, France Emails: Henriette Poaty- [email protected] Fanny Pelluard - [email protected] Gwenaelle André- [email protected] Brigitte Maugey-Laulom- [email protected] Dominique Carles- [email protected] Abstract Background: Truncus arteriosus communis (TAC) is a congenital heart defect in which the physiologic arterial common trunk was not divided into aorta and pulmonary artery trunk. Objectives: In this paper, we report on three observed cases from which we looked for (in conjunction with literature review) the different causes of TAC many of which have genetic origins. Methods: We collected three clinical files of fetuses having a TAC. Two of them were examinated after a medical termination of pregnancy motivated by severe cardiopathy. The malformation had been diagnosed based on different techniques: echocardi- ography, skeletal radiography, arteriography, fetal autopsy, karyotype and fluorescence in situ hybridization (FISH). Results: Imaging and fetopathological examination revealed the presence of TAC type 3 and 4 in the Van Praaghs classification. FISH analysis showed a 22q11.2 deletion in one fetus in favour of Digeorge syndrome. The karyotype analysis performed in two cases was normal. Conclusion: Truncus arteriosus is a rare pathology caused by numerous etiologies from which many of them have genetic ori- gin. -

Anatomy of the Heart
Anatomy of the Heart DR. SAEED VOHRA DR. SANAA AL-SHAARAWI OBJECTIVES • At the end of the lecture, the student should be able to : • Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. • Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. • List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. • Describe the innervation of the heart • Briefly describe the conduction system of the Heart The Heart • It lies in the middle mediastinum. • It is surrounded by a fibroserous sac called pericardium which is differentiated into an outer fibrous layer (Fibrous pericardium) & inner serous sac (Serous pericardium). • The Heart is somewhat pyramidal in shape, having: • Apex • Sterno-costal (anterior surface) • Base (posterior surface). • Diaphragmatic (inferior surface) • It consists of 4 chambers, 2 atria (right& left) & 2 ventricles (right& left) Apex of the heart • Directed downwards, forwards and to the left. • It is formed by the left ventricle. • Lies at the level of left 5th intercostal space 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface Sterno-costal (anterior)surface • Divided by coronary (atrio- This surface is formed mainly ventricular) groove into : by the right atrium and the right . Atrial part, formed mainly by ventricle right atrium. Ventricular part , the right 2/3 is formed by right ventricle, while the left l1/3 is formed by left ventricle. -

The Functional Anatomy of the Heart. Development of the Heart, Anomalies
The functional anatomy of the heart. Development of the heart, anomalies Anatomy and Clinical Anatomy Department Anastasia Bendelic Plan: Cardiovascular system – general information Heart – functional anatomy Development of the heart Abnormalities of the heart Examination in a living person Cardiovascular system Cardiovascular system (also known as vascular system, or circulatory system) consists of: 1. heart; 2. blood vessels (arteries, veins, capillaries); 3. lymphatic vessels. Blood vessels Arteries are blood vessels that carry blood away from the heart. Veins carry blood back towards the heart. Capillaries are tiny blood vessels, that connect arteries to veins. Lymphatic vessels: lymphatic capillaries; lymphatic vessels (superficial and deep lymph vessels); lymphatic trunks (jugular, subclavian, bronchomediastinal, lumbar, intestinal trunks); lymphatic ducts (thoracic duct and right lymphatic duct). Lymphatic vessels Microcirculation Microcirculatory bed comprises 7 components: 1. arterioles; 2. precapillaries or precapillary arterioles; 3. capillaries; 4. postcapillaries or postcapillary venules; 5. venules; 6. lymphatic capillaries; 7. interstitial component. Microcirculation The heart Heart is shaped as a pyramid with: an apex (directed downward, forward and to the left); a base (facing upward, backward and to the right). There are four surfaces of the heart: sternocostal (anterior) surface; diaphragmatic (inferior) surface; right pulmonary surface; left pulmonary surface. External surface of the heart The heart The heart has four chambers: right and left atria; right and left ventricles. Externally, the atria are demarcated from the ventricles by coronary sulcus (L. sulcus coronarius). The right and left ventricles are demarcated from each other by anterior and posterior interventricular sulci (L. sulci interventriculares anterior et posterior). Chambers of the heart The atria The atria are thin-walled chambers, that receive blood from the veins and pump it into the ventricles. -

Regulation of the Aortic Valve Opening
REGULATION OF THE Aortic valve orifice area was dynamically measured in anesthetized dogs AORTIC VALVE OPENING with a new measuring system involving electromagnetic induction. This system permits us real-time measurement of the valve orifice area in In vivo dynamic beating hearts in situ. The aortic valve was already open before aortic measurement of aortic pressure started to increase without detectable antegrade aortic flow. valve orifice area Maximum opening area was achieved while flow was still accelerating at a mean of 20 to 35 msec before peak blood flow. Maximum opening area was affected by not only aortic blood flow but also aortic pressure, which produced aortic root expansion. The aortic valve orifice area's decreasing curve (corresponding to valve closure) was composed of two phases: the initial decrease and late decrease. The initial decrease in aortic valve orifice area was slower (4.1 cm2/sec) than the late decrease (28.5 cm2/sec). Aortic valve orifice area was reduced from maximum to 40% of maximum (in a triangular open position) during the initial slow closing. These measure- ments showed that (1) initial slow closure of the aortic valve is evoked by leaflet tension which is produced by the aortic root expansion (the leaflet tension tended to make the shape of the aortic orifice triangular) and (2) late rapid closure is induced by backflow of blood into the sinus of Valsalva. Thus, cusp expansion owing to intraaortic pressure plays an important role in the opening and closing of the aortic valve and aortic blood flow. (J THORAC CARDIOVASC SURG 1995;110:496-503) Masafumi Higashidate, MD, a Kouichi Tamiya, MD, b Toshiyuki Beppu, MS, b and Yasuharu Imai, MD, a Tokyo, Japan n estimation of orifice area of the aortic valve is ers, 4 as well as echocardiography. -
Embryo a the Development of the Heart
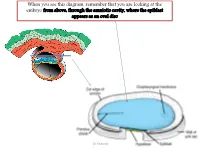
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc Dr. Shatarat Dr. Shatarat Why the embryo needs the vascular system? When it appears? Where it appears? with later contributions from neural crest mesenchyme Dr. Shatarat The Cardiac progenitor cells migrate from the Epiblast Through In a Cranial direction on Primitive streak each side of the notochordal process and around the prechordal plate 4 Dr. Shatarat into the splanchnic layer of the lateral plate mesoderm Dr. Shatarat into the splanchnic layer of the lateral plate mesoderm Dr. Shatarat The cells from both sides meet cranially to form the Primary Heart Field (PHF) These cells will form : • The atria • Left ventricle • Part of right ventricle • The remainder of the right ventricle • outflow tract (conus cordis and truncus arteriosus) Are derived from the Secondary Heart Field (SHF) Dr. Shatarat ONE-SOMITE AND TWO-SOMITE STAGES Dr. Shatarat Paired endothelial strands ANGIOBLASTIC CORDS appear in the cardiogenic mesoderm during the third week of development Dr. Shatarat Dr. Shatarat These cords canalize to form two heart tubes that soon fuse as embryo folds laterally to form a single heart tube late in the third week Two hearts tubs The two tubs are Fused Single heart tube is formed As the embryo folds laterally Dr. Shatarat Dr. Shatarat In addition to the cardiogenic region, other blood islands appear bilaterally, parallel and close to the midline of the embryonic shield. These islands form a pair of longitudinal vessels, the dorsal aortae. -

1-Anatomy of the Heart.Pdf
Color Code Important Anatomy of the Heart Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the student should be able to : ✓Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. ✓Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. ✓List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. ✓Describe the innervation of the heart. ✓Briefly describe the conduction system of the Heart. The Heart o It lies in the middle mediastinum. o It consists of 4 chambers: • 2 atria (right& left) that receive blood & • 2 ventricles (right& left) that pump blood. o The Heart is somewhat pyramidal in shape, having: 1. Apex Extra 2. Sterno-costal (anterior surface) 3. Diaphragmatic (inferior surface) 4. Base (posterior surface). o It is surrounded by a fibroserous sac called pericardium which is differentiated into: an outer fibrous layer inner serous sac (Fibrous pericardium) (Serous pericardium). further divided into Parietal Visceral (Epicardium) The Heart Extra Extra The Heart 1- Apex o Directed downwards, forwards and to the left. o It is formed by the left ventricle. o Lies at the level of left 5th intercostal space (the same level of the nipple) 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite to the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface. -

Unit 1 Anatomy of the Heart
UNIT 1 ANATOMY OF THE HEART Structure 1.0 Objectives 1.1 Introduction 1.2 Chambers of Heart 1.3 Orifices of Heart 1.4 The Conducting System of the Heart 1.5 Blood Supply of the Heart 1.6 Surface Markings of the Heart 1.7 Let Us Sum Up 1.8 Answers to Check Your Progress 1.0 OBJECTIVES After reading this unit, you should be able to: • understand the proper position of a heart inside the thorax; • know the various anatomical structures of various chambers of heart and various valves of heart; • know the arterial supply, venous drainage and lymphatic drainage of heart; and • describe the surface marking of heart. 1.1 INTRODUCTION The human heart is a cone-shaped, four-chambered muscular pump located in the mediastinal cavity of the thorax between the lungs and beneath the sternum, designed to ensure the circulation through the tissues of the body. The cone-shaped heart lies on its side on the diaphragm, with its base (the widest part) upward and leaning toward the right shoulder, and its apex pointing down and to the left. Structurally and functionally it consists of two halves–right and left. The right heart circulates blood only through the lungs for the purpose of pulmonary circulation. The left heart sends blood to tissues of entire body/systemic circulation. The heart is contained in a sac called the pericardium. The four chambers are right and left atria and right and left ventricles. The heart lies obliquely across the thorax and the right side is turned to face the front. -

Findings from Chick Embryo Studies
Journal of Cardiovascular Development and Disease Review Mechanosensitive Pathways in Heart Development: Findings from Chick Embryo Studies Maha Alser 1,2, Samar Shurbaji 1 and Huseyin C. Yalcin 1,* 1 Biomedical Research Center, Qatar University, Doha P.O. Box 2713, Qatar; [email protected] (M.A.); [email protected] (S.S.) 2 College of Health and Life Sciences, Hamad Bin Khalifa University, Doha P.O. Box 34110, Qatar * Correspondence: [email protected]; Tel.: +974-4403-7719 Abstract: The heart is the first organ that starts to function in a developing embryo. It continues to undergo dramatic morphological changes while pumping blood to the rest of the body. Genetic regulation of heart development is partly governed by hemodynamics. Chick embryo is a major animal model that has been used extensively in cardiogenesis research. To reveal mechanosensitive pathways, a variety of surgical interferences and chemical treatments can be applied to the chick embryo to manipulate the blood flow. Such manipulations alter expressions of mechanosensitive genes which may anticipate induction of morphological changes in the developing heart. This paper aims to present different approaches for generating clinically relevant disturbed hemodynamics conditions using this embryonic chick model and to summarize identified mechanosensitive genes using the model, providing insights into embryonic origins of congenital heart defects. Keywords: chick embryo; hemodynamics; mechanobiology; mechanosensitive pathways; surgical interferences; left atrial ligation; outflow tract banding; vitelline vein ligation; chemical treatment; gene expression Citation: Alser, M.; Shurbaji, S.; Yalcin, H.C. Mechanosensitive Pathways in Heart Development: Findings from Chick Embryo Studies. 1. Introduction J. Cardiovasc. Dev. -

6. Heart and Circulatory System I
6. HEART AND CIRCULATORY SYSTEM I Dr. Taube P. Rothman P&S 12-520 [email protected] 212-305-7930 RECOMMENDED READING: Larsen Human Embryology, 3rd Edition, pp. 195-199; 157-169 top left; 172-174; bottom 181-182; 187-top 189, Simbryo-cardiovascular system SUMMARY: The circulatory system, consisting of heart, blood vessels, and blood cells is the first functional organ to develop. This lecture will focus on the formation of the embryonic vasculature, the origin and formation of the early heart tube and primitive cardiac chambers, cardiac looping, and the primitive circulation. Between the 5th - 8th week of embryonic development, the tubular heart is remodeled into a four chambered structure. We will see how right and left atrioventricular canals connect each atrium with its respective ventricle, and how the atrial septum and definitive right and left atria form. We will also see why the great veins deliver blood to the right atrium while the pulmonary veins empty into the left. GLOSSARY: Angioblasts: precursors of blood vessels Angiogenesis: lengthening, branching, sprouting and remodeling of embryonic blood vessels Aortic arches: paired arteries surrounding the pharynx; portions will contribute to formation of the great arterial vessels Blood islands: clusters of cells in the yolk sac, connecting stalk and chorionic villi that form primitive blood vessels Cardiac jelly: gelatinous extracellular matrix that forms the middle layer of the heart tube Ductus venosus: shunts most of the blood in the umbilical vein into the inferior vena cava