Findings from Chick Embryo Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

TBX20 Loss-Of-Function Mutation Contributes to Double Outlet Right Ventricle
1058 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 35: 1058-1066, 2015 TBX20 loss-of-function mutation contributes to double outlet right ventricle YUN PAN1*, RUI GENG1*, NING ZHOU1, GUI-FEN ZHENG1, HONG ZHAO1, JUAN WANG2, CUI-MEI ZHAO2, XING-BIAO QIU3, YI-QING YANG3-5 and XING-YUAN LIU1 Departments of 1Pediatrics and 2Cardiology, Tongji Hospital, Tongji University School of Medicine, Shanghai 200065; 3Department of Cardiology, 4Cardiovascular Research Laboratory and 5Central Laboratory, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai 200030, P.R. China Received November 30, 2014; Accepted January 20, 2015 DOI: 10.3892/ijmm.2015.2077 Abstract. Congenital heart disease (CHD), the most prevalent nant pattern with complete penetrance. The missense mutation birth defect in humans worldwide, is still a leading non-infec- was absent in 400 control chromosomes and the altered amino tious cause of infant morbidity and mortality. Increasing acid was completely conserved evolutionarily across species. evidence demonstrates that genetic risk factors play a key role Functional analysis revealed that mutant TBX20 had a signifi- in the pathogenesis of CHD, and more than 50 genes have cantly diminished transcriptional activity compared with its been linked to various types of CHD. Nevertheless, CHD is wild-type counterpart. To the best of our knowledge, this study a heterogeneous disorder and the genetic components under- is the first to report the association of TBX20 loss-of-function pinning CHD in an overwhelming majority of cases remain mutation with increased susceptibility to DORV in humans, unknown. In the present study, the entire coding exons and which provides novel insight into the molecular mechanisms flanking introns of the TBX20 gene, which codes for a T-box responsible for CHD, suggesting potential implications for the transcription factor essential for the proper development of the antenatal prophylaxis of CHD. -

When Does Human Life Begin? the Scientific Evidence and Terminology Revisited
University of St. Thomas Journal of Law and Public Policy Volume 8 Issue 1 Fall 2013 Article 4 January 2013 When Does Human Life Begin? The Scientific videnceE and Terminology Revisited Maureen L. Condic Follow this and additional works at: https://ir.stthomas.edu/ustjlpp Part of the Family Law Commons, and the Health Law and Policy Commons Recommended Citation Maureen L. Condic, When Does Human Life Begin? The Scientific videnceE and Terminology Revisited, 8 U. ST. THOMAS J.L. & PUB. POL'Y 44 (2013). Available at: https://ir.stthomas.edu/ustjlpp/vol8/iss1/4 This Article is brought to you for free and open access by UST Research Online and the University of St. Thomas Journal of Law and Public Policy. For more information, please contact the Editor-in-Chief at [email protected]. WHEN DOES HUMAN LIFE BEGIN? THE SCIENTIFIC EVIDENCE AND TERMINOLOGY REVISITED MAUREEN L. CONDIC* The question of when human life begins continues to be a source of ethical and political controversy. In this debate, the language used by many medical textbooks fosters significant misinterpretation of the scientific facts. In particular, terminology that refers to the product of sperm-egg fusion as a "penetrated oocyte" and claims that the zygote does not form until syngamy (approximately twenty-four hours after sperm-egg fusion) have resulted in the erroneous belief that a human embryo does not exist during the period prior to this point (i.e. the "pre-zygote error"). Yet an objective view of the modem scientific evidence supports only a single definition of the term "zygote": a one-cell human organism (i.e. -

Introduction – Developmental Overview of the Human Embryo
1 Introduction – Developmental Overview of the Human Embryo Shigehito Yamada1, and Tetsuya Takakuwa2 1Congenital Anomaly Research Center, Kyoto University, 2Human Health Science, Kyoto University, Japan 1. Introduction In this chapter, we provide a historical background on human embryo collections and describe their significant contribution to the understanding of human ontogenesis. More particularly, an overview of human embryonic development is presented using computer- generated images obtained from embryonic specimens housed at the Kyoto Collection in Japan. 1.1 Human embryology and embryo collections Historically, several human embryo collections have been created. The Carnegie Collection, the Blechschmidt Collection, the Hinrichsen Collection and the Kyoto Collection are reported as the four famous compendiums of human embryos in the world. The Carnegie Collection is the oldest and was established as early as 1887, while the Blechschmidt collection was created in 1948 by the Göttingen anatomist Erich Blechschmidt, well known for its contribution to the development of novel methods of reconstruction. In 1961, the Kyoto Collection of Human Embryos was instigated, followed by the Hinrichsen Collection in 1969. While the Blechschmidt and the Hinrichsen collections are described in Chapter 2, here, we focus on the Carnegie and the Kyoto Collections. 1.2 The carnegie human embryo collection The basis of the Carnegie Human Embryo Collection was established by Franklin P. Mall. After earning his medical degree at the University of Michigan in 1883, Mall traveled to Germany to receive a clinical training and there he met Wilhelm His and other eminent biologists. Mall then became aware of the importance of studying human embryology, and initiated a collection of human embryos in 1887. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Truncus Arteriosus Communis: Report of Three Cases and Review of Literature
Truncus arteriosus communis: report of three cases and review of literature Henriette Poaty1,2, Fanny Pelluard3, Gwenaelle André3, Brigitte Maugey-Laulom4, Dominique Carles3 1. Histology-Embryology and Genetic Laboratory, Faculty of Health Sciences, BP 2672, Marien Ngouabi University, Brazzaville, Congo. 2. National Research Institute on Health Sciences, Brazzaville, Congo 3. Department of Fetopathology, CHU Pellegrin, place Amélie Raba, 33076 Bordeaux cedex France 4. Fetal Imaging Unit, Maternity, CHU Pellegrin, place Amélie Raba, 33076 Bordeaux, France Emails: Henriette Poaty- [email protected] Fanny Pelluard - [email protected] Gwenaelle André- [email protected] Brigitte Maugey-Laulom- [email protected] Dominique Carles- [email protected] Abstract Background: Truncus arteriosus communis (TAC) is a congenital heart defect in which the physiologic arterial common trunk was not divided into aorta and pulmonary artery trunk. Objectives: In this paper, we report on three observed cases from which we looked for (in conjunction with literature review) the different causes of TAC many of which have genetic origins. Methods: We collected three clinical files of fetuses having a TAC. Two of them were examinated after a medical termination of pregnancy motivated by severe cardiopathy. The malformation had been diagnosed based on different techniques: echocardi- ography, skeletal radiography, arteriography, fetal autopsy, karyotype and fluorescence in situ hybridization (FISH). Results: Imaging and fetopathological examination revealed the presence of TAC type 3 and 4 in the Van Praaghs classification. FISH analysis showed a 22q11.2 deletion in one fetus in favour of Digeorge syndrome. The karyotype analysis performed in two cases was normal. Conclusion: Truncus arteriosus is a rare pathology caused by numerous etiologies from which many of them have genetic ori- gin. -

Myocardin Is Sufficient and Necessary for Cardiac Gene Expression in Xenopus Eric M
Research article 987 Myocardin is sufficient and necessary for cardiac gene expression in Xenopus Eric M. Small1,*,†, Andrew S. Warkman1,*, Da-Zhi Wang2, Lillian B. Sutherland3, Eric N. Olson3 and Paul A. Krieg1,‡ 1Department of Cell Biology and Anatomy, University of Arizona Health Sciences Center, 1501 N. Campbell Avenue, PO Box 245044, Tucson, AZ, 85724, USA 2Carolina Cardiovascular Biology Center, Department of Cell and Developmental Biology, University of North Carolina, Chapel Hill, NC 27599-7126, USA 3Department of Molecular Biology, University of Texas Southwestern Medical Center, 6000 Harry Hines Boulevard, Dallas, TX 75390-9148, USA *These authors contributed equally to this work †Present address: Cardiovascular Research, The Hospital for Sick Children, 555 University Avenue, Toronto, ON M5G 1X8, Canada ‡Author for correspondence (e-mail: [email protected]) Accepted 14 December 2004 Development 132, 987-997 Published by The Company of Biologists 2005 doi:10.1242/dev.01684 Summary Myocardin is a cardiac- and smooth muscle-specific co- expression. Inhibition of myocardin activity in Xenopus factor for the ubiquitous transcription factor serum embryos using morpholino knockdown methods results in response factor (SRF). Using gain-of-function approaches inhibition of cardiac development and the absence of in the Xenopus embryo, we show that myocardin is expression of cardiac differentiation markers and severe sufficient to activate transcription of a wide range of disruption of cardiac morphological processes. We cardiac and -

MOCHI Enables Discovery of Heterogeneous Interactome Modules in 3D Nucleome
Downloaded from genome.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press MOCHI enables discovery of heterogeneous interactome modules in 3D nucleome Dechao Tian1,# , Ruochi Zhang1,# , Yang Zhang1, Xiaopeng Zhu1, and Jian Ma1,* 1Computational Biology Department, School of Computer Science, Carnegie Mellon University, Pittsburgh, PA 15213, USA #These two authors contributed equally *Correspondence: [email protected] Contact To whom correspondence should be addressed: Jian Ma School of Computer Science Carnegie Mellon University 7705 Gates-Hillman Complex 5000 Forbes Avenue Pittsburgh, PA 15213 Phone: +1 (412) 268-2776 Email: [email protected] 1 Downloaded from genome.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press Abstract The composition of the cell nucleus is highly heterogeneous, with different constituents forming complex interactomes. However, the global patterns of these interwoven heterogeneous interactomes remain poorly understood. Here we focus on two different interactomes, chromatin interaction network and gene regulatory network, as a proof-of-principle, to identify heterogeneous interactome modules (HIMs), each of which represents a cluster of gene loci that are in spatial contact more frequently than expected and that are regulated by the same group of transcription factors. HIM integrates transcription factor binding and 3D genome structure to reflect “transcriptional niche” in the nucleus. We develop a new algorithm MOCHI to facilitate the discovery of HIMs based on network motif clustering in heterogeneous interactomes. By applying MOCHI to five different cell types, we found that HIMs have strong spatial preference within the nucleus and exhibit distinct functional properties. Through integrative analysis, this work demonstrates the utility of MOCHI to identify HIMs, which may provide new perspectives on the interplay between transcriptional regulation and 3D genome organization. -

Discovery and Systematic Characterization of Risk Variants and Genes For
medRxiv preprint doi: https://doi.org/10.1101/2021.05.24.21257377; this version posted June 2, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY 4.0 International license . 1 Discovery and systematic characterization of risk variants and genes for 2 coronary artery disease in over a million participants 3 4 Krishna G Aragam1,2,3,4*, Tao Jiang5*, Anuj Goel6,7*, Stavroula Kanoni8*, Brooke N Wolford9*, 5 Elle M Weeks4, Minxian Wang3,4, George Hindy10, Wei Zhou4,11,12,9, Christopher Grace6,7, 6 Carolina Roselli3, Nicholas A Marston13, Frederick K Kamanu13, Ida Surakka14, Loreto Muñoz 7 Venegas15,16, Paul Sherliker17, Satoshi Koyama18, Kazuyoshi Ishigaki19, Bjørn O Åsvold20,21,22, 8 Michael R Brown23, Ben Brumpton20,21, Paul S de Vries23, Olga Giannakopoulou8, Panagiota 9 Giardoglou24, Daniel F Gudbjartsson25,26, Ulrich Güldener27, Syed M. Ijlal Haider15, Anna 10 Helgadottir25, Maysson Ibrahim28, Adnan Kastrati27,29, Thorsten Kessler27,29, Ling Li27, Lijiang 11 Ma30,31, Thomas Meitinger32,33,29, Sören Mucha15, Matthias Munz15, Federico Murgia28, Jonas B 12 Nielsen34,20, Markus M Nöthen35, Shichao Pang27, Tobias Reinberger15, Gudmar Thorleifsson25, 13 Moritz von Scheidt27,29, Jacob K Ulirsch4,11,36, EPIC-CVD Consortium, Biobank Japan, David O 14 Arnar25,37,38, Deepak S Atri39,3, Noël P Burtt4, Maria C Costanzo4, Jason Flannick40, Rajat M 15 Gupta39,3,4, Kaoru Ito18, Dong-Keun Jang4, -
Embryo a the Development of the Heart
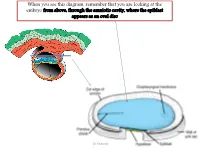
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc Dr. Shatarat Dr. Shatarat Why the embryo needs the vascular system? When it appears? Where it appears? with later contributions from neural crest mesenchyme Dr. Shatarat The Cardiac progenitor cells migrate from the Epiblast Through In a Cranial direction on Primitive streak each side of the notochordal process and around the prechordal plate 4 Dr. Shatarat into the splanchnic layer of the lateral plate mesoderm Dr. Shatarat into the splanchnic layer of the lateral plate mesoderm Dr. Shatarat The cells from both sides meet cranially to form the Primary Heart Field (PHF) These cells will form : • The atria • Left ventricle • Part of right ventricle • The remainder of the right ventricle • outflow tract (conus cordis and truncus arteriosus) Are derived from the Secondary Heart Field (SHF) Dr. Shatarat ONE-SOMITE AND TWO-SOMITE STAGES Dr. Shatarat Paired endothelial strands ANGIOBLASTIC CORDS appear in the cardiogenic mesoderm during the third week of development Dr. Shatarat Dr. Shatarat These cords canalize to form two heart tubes that soon fuse as embryo folds laterally to form a single heart tube late in the third week Two hearts tubs The two tubs are Fused Single heart tube is formed As the embryo folds laterally Dr. Shatarat Dr. Shatarat In addition to the cardiogenic region, other blood islands appear bilaterally, parallel and close to the midline of the embryonic shield. These islands form a pair of longitudinal vessels, the dorsal aortae. -

Human Development Domain of the Ontology of Craniofacial
Human Development Domain of the Ontology of Craniofacial Development and Malformation Jose LV Mejino Jr.1.*, Ravensara S Travillian1,2,, Timothy C Cox3,4,5., Linda G Shapiro1,2,6 and James F Brinkley1,2 1 Structural Informatics Group, Department of Biological Structure, University of Washington, Seattle, WA USA 2 Department of Biomedical Informatics and Medical Education, University of Washington, Seattle, WA USA 3 Division of Craniofacial Medicine, Department of Pediatrics, University of Washington, Seattle, WA USA 4 Center for Tissue and Cell Sciences, Seattle Children’s Research Institute; Seattle, WA USA 5Department of Anatomy & Developmental Biology, Monash University, Clayton, Victoria, Australia 6Department of Computer Science & Engineering, University of Washington, Seattle, WA USA ABSTRACT computer-processable way. The ATA component of the In this paper we describe an ontological scheme for representing ana- FMA was not built as part of the original ontology, but a tomical entities undergoing morphological transformation and changes in phenotype during prenatal development. This is a proposed component of need for it has come up in our current work on the design the Anatomical Transformation Abstraction (ATA) of the Foundational and implementation of the Ontology of Craniofacial Devel- Model of Anatomy (FMA) Ontology that was created to provide an onto- opment and Malformation (OCDM), which we are building logical framework for capturing knowledge about human development from the zygote to postnatal life. It is designed to initially describe the as part of our NIDCR-sponsored project for the FaceBase structural properties of the anatomical entities that participate in human Consortium (https://www.facebase.org/) [4], which deals development and then enhance their description with developmental prop- with craniofacial abnormalities. -

Embryo Makes Contact with the Endometrial Lining of the Uterus
Week 1 • Week 1 - Early zygote • Stage 1 starts at the beginning of • Week 1 Carnegie stage – 1,2,3,4, fertilization • Fertilization • Stage 2 begins with the division • of the zygote into two cells and Zygote ends with the appearance of the • Morula blastocystic cavity • Blastocyst • Stage 3 begins when the blastocystic cavity first appears in the morula and ends when the zona (capsula) pellucida is shed as the embryo makes contact with the endometrial lining of the uterus. • Stage 4 is reserved for the attaching blastocyst to the endometrial lining Week 2 • Week 2 Implantation • Stage 5 Two distinct layers • Week 2 Carnegie stage -5,6 are evident in the • Trophoblast - outer cell trophoblast; 1) a thicker layer outer layer without cell boundaries, called the • Embryoblast - inner cell syncytiotrophoblast and 2) mass a thinner inner layer with • Implantation cell boundaries called the • Bilaminar embryo cytotrophoblast. • Stage 6 the first appearance of chorionic villi. Week 3 • • Stage 7 the presomite • Week 3 - Embryonic disc period and well defined • Week 3 - Carnegie stage – embryonic disc appearance 7,8, &9 of the notochordal process and the gastrulation • Gastrulation (primitive) node. • Notochord formation • Trilaminar embryo • Mesoderm • Somitogenesis • Neurogenesis Week 4 • The heart begins • Week 4 - Carnegie stage -10,11,12 &13 • Heart • Placodes • Pharyngeal arches • Week 5 • Week 7 - Head and limb • Carnegie stages stage 14 development stage 15 • Carnegie stages stage 18 • stage 19 • Week 6 - Early face • Week 8 deevelopment • Last embryonic stage • Carnegie stages Carnegie stage – 20 21 22 • Week 6 - Carnegie stage 16 &23 & 17 • Last week of embryonic development.