Tuft Cells from the Mucosa to the Thymus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adaptive Immune Systems
Immunology 101 (for the Non-Immunologist) Abhinav Deol, MD Assistant Professor of Oncology Wayne State University/ Karmanos Cancer Institute, Detroit MI Presentation originally prepared and presented by Stephen Shiao MD, PhD Department of Radiation Oncology Cedars-Sinai Medical Center Disclosures Bristol-Myers Squibb – Contracted Research What is the immune system? A network of proteins, cells, tissues and organs all coordinated for one purpose: to defend one organism from another It is an infinitely adaptable system to combat the complex and endless variety of pathogens it must address Outline Structure of the immune system Anatomy of an immune response Role of the immune system in disease: infection, cancer and autoimmunity Organs of the Immune System Major organs of the immune system 1. Bone marrow – production of immune cells 2. Thymus – education of immune cells 3. Lymph Nodes – where an immune response is produced 4. Spleen – dual role for immune responses (especially antibody production) and cell recycling Origins of the Immune System B-Cell B-Cell Self-Renewing Common Progenitor Natural Killer Lymphoid Cell Progenitor Thymic T-Cell Selection Hematopoetic T-Cell Stem Cell Progenitor Dendritic Cell Myeloid Progenitor Granulocyte/M Macrophage onocyte Progenitor The Immune Response: The Art of War “Know your enemy and know yourself and you can fight a hundred battles without disaster.” -Sun Tzu, The Art of War Immunity: Two Systems and Their Key Players Adaptive Immunity Innate Immunity Dendritic cells (DC) B cells Phagocytes (Macrophages, Neutrophils) Natural Killer (NK) Cells T cells Dendritic Cells: “Commanders-in-Chief” • Function: Serve as the gateway between the innate and adaptive immune systems. -

Cells, Tissues and Organs of the Immune System
Immune Cells and Organs Bonnie Hylander, Ph.D. Aug 29, 2014 Dept of Immunology [email protected] Immune system Purpose/function? • First line of defense= epithelial integrity= skin, mucosal surfaces • Defense against pathogens – Inside cells= kill the infected cell (Viruses) – Systemic= kill- Bacteria, Fungi, Parasites • Two phases of response – Handle the acute infection, keep it from spreading – Prevent future infections We didn’t know…. • What triggers innate immunity- • What mediates communication between innate and adaptive immunity- Bruce A. Beutler Jules A. Hoffmann Ralph M. Steinman Jules A. Hoffmann Bruce A. Beutler Ralph M. Steinman 1996 (fruit flies) 1998 (mice) 1973 Discovered receptor proteins that can Discovered dendritic recognize bacteria and other microorganisms cells “the conductors of as they enter the body, and activate the first the immune system”. line of defense in the immune system, known DC’s activate T-cells as innate immunity. The Immune System “Although the lymphoid system consists of various separate tissues and organs, it functions as a single entity. This is mainly because its principal cellular constituents, lymphocytes, are intrinsically mobile and continuously recirculate in large number between the blood and the lymph by way of the secondary lymphoid tissues… where antigens and antigen-presenting cells are selectively localized.” -Masayuki, Nat Rev Immuno. May 2004 Not all who wander are lost….. Tolkien Lord of the Rings …..some are searching Overview of the Immune System Immune System • Cells – Innate response- several cell types – Adaptive (specific) response- lymphocytes • Organs – Primary where lymphocytes develop/mature – Secondary where mature lymphocytes and antigen presenting cells interact to initiate a specific immune response • Circulatory system- blood • Lymphatic system- lymph Cells= Leukocytes= white blood cells Plasma- with anticoagulant Granulocytes Serum- after coagulation 1. -

Urology & Incontinence
Urology & Incontinence - Glossary of Terms Anti-reflux Refers to a tube or collapsible material within a urine collection device to help prevent urine from reentering the tubing. Applicator collar Found on some Hollister male external catheters, a plastic guide with notches for the thumb and forefinger to assist proper placement against the tip of the penis. Aseptic intermittent catheterization The process of performing intermittent catheterization using sterile equipment and inserting the catheter in a sterile way. This would include a sterile ready-to-use product that can be inserted with gloves using a no-touch technique (e.g., the Advance Plus intermittent catheter or a VaPro hydrophilic catheter). Benzalkonium chloride (BZK) An antimicrobial solution used for cleansing the urethral opening prior to inserting an intermittent catheter. Does not stain skin or clothing. Bladder A collapsible balloon-like muscular organ that lies in the pelvis and functions to store and expel urine. Bladder catheterization A procedure in which a catheter is passed through the urethra or stoma into the bladder, usually for the purpose of draining urine. Bladder control The ability to control urination. Bladder diary A printed or electronic form to keep track of when one urinates or leaks urine. Catheter (urinary) A special type of hollow tube inserted through the urethra or a stoma to the bladder to withdraw urine or instill medication. Catheterization The process of inserting a tube into the bladder to drain urine. Clean intermittent catheterization The process of emptying the bladder using a clean intermittent catheter. It involves inserting and removing a catheter, typically several times a day. -
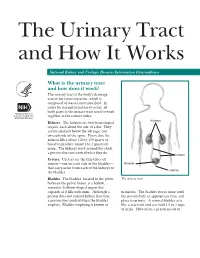
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

15-1040-Junu Oh-Neuronal.Key
Neuronal Control of the Bladder Seung-June Oh, MD Department of urology, Seoul National University Hospital Seoul National University College of Medicine Contents Relevant end organs and nervous system Reflex pathways Implication in the sacral neuromodulation Urinary bladder ! body: detrusor ! trigone and bladder neck Urethral sphincters B Preprostatic S Smooth M. Sphincter Passive Prostatic S Skeletal M. Sphincter P Prostatic SS P-M Striated Sphincter Membraneous SS Periurethral Striated M. Pubococcygeous Spinal cord ! S2–S4 spinal cord ! primary parasympathetic micturition center ! bladder and distal urethral sphincter ! T11-L2 spinal cord ! sympathetic outflow ! bladder and proximal urethral sphincter Peripheral innervation ! The lower urinary tract is innervated by 3 principal sets of peripheral nerves: ! parasympathetic -pelvic n. ! sympathetic-hypogastric n. ! somatic nervous systems –pudendal n. ! Parasympathetic and sympathetic nervous systems form pelvic plexus at the lateral side of the rectum before reaching bladder and sphincter Sympathetic & parasympathetic systems ! Sympathetic pathways ! originate from the T11-L2 (sympathetic nucleus; intermediolateral column of gray matter) ! inhibiting the bladder body and excite the bladder base and proximal urethral sphincter ! Parasympathetic nerves ! emerge from the S2-4 (parasympathetic nucleus; intermediolateral column of gray matter) ! exciting the bladder and relax the urethra Sacral somatic system !emerge from the S2-4 (Onuf’s nucleus; ventral horn) !form pudendal nerve, providing -

Under the Human Keratin 14 Promoter Expressed Autoimmune Disease to an Antigen a Spontaneous CD8 T Cell-Dependent
A Spontaneous CD8 T Cell-Dependent Autoimmune Disease to an Antigen Expressed Under the Human Keratin 14 Promoter This information is current as Maureen A. McGargill, Dita Mayerova, Heather E. Stefanski, of September 26, 2021. Brent Koehn, Evan A. Parke, Stephen C. Jameson, Angela Panoskaltsis-Mortari and Kristin A. Hogquist J Immunol 2002; 169:2141-2147; ; doi: 10.4049/jimmunol.169.4.2141 http://www.jimmunol.org/content/169/4/2141 Downloaded from References This article cites 44 articles, 23 of which you can access for free at: http://www.jimmunol.org/content/169/4/2141.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 26, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2002 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology A Spontaneous CD8 T Cell-Dependent Autoimmune Disease to an Antigen Expressed Under the Human Keratin 14 Promoter1 Maureen A. McGargill,*‡ Dita Mayerova,*‡ Heather E. -

Nerve Disease and Bladder Control
Nerve Disease and Bladder Control National Kidney and Urologic Diseases Information Clearinghouse For the urinary system to do its job, muscles and nerves must work together to hold Brain urine in the bladder and then release it at the right time. Nerves carry messages from NATIONAL the bladder to the brain to let it know when INSTITUTES the bladder is full. They also carry messages OF HEALTH Central nervous from the brain to the bladder, telling muscles system (brain either to tighten or release. A nerve prob and spinal cord) lem might affect your bladder control if the nerves that are supposed to carry messages Spinal cord between the brain and the bladder do not work properly. Nerve signals U.S. Department to bladder of Health and Bladder and sphincter Human Services What bladder control muscles problems does nerve damage cause? Nerves that work poorly can lead to three Urethra different kinds of bladder control problems. Overactive bladder. Damaged nerves may Sphincter muscles send signals to the bladder at the wrong time, causing its muscles to squeeze with Nerves carry signals from the brain to the bladder out warning. The symptoms of overactive and sphincter. bladder include • urinary frequency—defined as urination eight or more times a day or two or nerves to the sphincter muscles are dam more times at night aged, the muscles may become loose and allow leakage or stay tight when you are • urinary urgency—the sudden, strong trying to release urine. need to urinate immediately Urine retention. For some people, nerve • urge incontinence—leakage of urine damage means their bladder muscles do that follows a sudden, strong urge to not get the message that it is time to release urinate urine or are too weak to completely empty Poor control of sphincter muscles. -
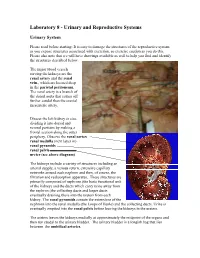
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Thymus Growth an Epithelial Progenitor Pool Regulates
An Epithelial Progenitor Pool Regulates Thymus Growth William E. Jenkinson, Andrea Bacon, Andrea J. White, Graham Anderson and Eric J. Jenkinson This information is current as of October 1, 2021. J Immunol 2008; 181:6101-6108; ; doi: 10.4049/jimmunol.181.9.6101 http://www.jimmunol.org/content/181/9/6101 Downloaded from References This article cites 35 articles, 16 of which you can access for free at: http://www.jimmunol.org/content/181/9/6101.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on October 1, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology An Epithelial Progenitor Pool Regulates Thymus Growth1 William E. Jenkinson, Andrea Bacon, Andrea J. White, Graham Anderson, and Eric J. Jenkinson2 Thymic epithelium provides an essential cellular substrate for T cell development and selection. Gradual age-associated thymic atrophy leads to a reduction in functional thymic tissue and a decline in de novo T cell generation. -

Incomplete Bladder Emptying and Tips to Help
A guide for patients about incomplete bladder emptying and tips to help What is incomplete bladder emptying? When the bladder doesn't empty properly this may cause numerous problems. One of the problems is known as overflow incontinence. This is when the bladder doesn’t empty and urine leaks out. You may not get the message to go to the toilet either. The bladder never empties completely so some residue is normal. You may find it difficult to start to pass water and that even when you have started; the flow is weak and slow. You might find that you dribble after you have finished passing water. Perhaps you dribble urine all the time, even without noticing. What causes incomplete bladder emptying? Incomplete bladder emptying occurs when the muscles of the bladder are not able to squeeze properly to empty the bladder. This can happen in cases where there may have been nerve or muscle damage, perhaps caused by injury, surgery, or disease such as Parkinson's disease, Multiple Sclerosis and Spina Bifida. Medication can also cause problems with bladder emptying. In some cases, it can be due to an obstruction which is making it more difficult for you to empty your bladder. This obstruction can be caused by an enlarged prostate in men, a kidney stone blocking the urethra, constipation, or stricture of the urethra in either men or women, which makes it difficult for urine to flow out of the bladder outlet. If you are not able to empty completely, your bladder and its muscles may become floppy over time. -

Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence
http://dx.doi.org/10.3349/ymj.2015.56.3.648 Original Article pISSN: 0513-5796, eISSN: 1976-2437 Yonsei Med J 56(3):648-657, 2015 Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence Jae Young Choi,1* So Young Chun,2* Bum Soo Kim,1 Hyun Tae Kim,1 Eun Sang Yoo,1 Yun-Hee Shon,2 Jeong Ok Lim,2 Seok Joong Yun,3 Phil Hyun Song,4 Sung Kwang Chung,1 James J Yoo,2,5 and Tae Gyun Kwon1,2 1Department of Urology, School of Medicine, Kyungpook National University, Daegu; 2Joint Institute for Regenerative Medicine, Kyungpook National University Hospital, Daegu; 3Department of Urology, College of Medicine, Chungbuk National University, Cheongju; 4Department of Urology, College of Medicine, Yeungnam University, Daegu, Korea; 5Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA. Received: March 31, 2014 Purpose: Stem cell-based therapies represent new promises for the treatment of Revised: August 10, 2014 urinary incontinence. This study was performed to assess optimized cell passage Accepted: August 13, 2014 number, cell dose, therapeutic efficacy, feasibility, toxicity, and cell trafficking for Corresponding author: Dr. Tae Gyun Kwon, the first step of the pre-clinical evaluation of human amniotic fluid stem cell (hAF- Department of Urology, School of Medicine, Kyungpook National University, SC) therapy in a urinary incontinence animal model. Materials and Methods: 130 Dongdeok-ro, Jung-gu, The proper cell passage number was analyzed with hAFSCs at passages 4, 6, and Daegu 700-721, Korea. -

Successful Repair Using Thymus Pedicle Flap for Tracheoesophageal Fistula: a Case Report
Fukumoto et al. Surgical Case Reports (2018) 4:49 https://doi.org/10.1186/s40792-018-0458-8 CASEREPORT Open Access Successful repair using thymus pedicle flap for tracheoesophageal fistula: a case report Yoji Fukumoto*, Tomoyuki Matsunaga, Yuji Shishido, Masataka Amisaki, Yusuke Kono, Yuki Murakami, Hirohiko Kuroda, Tomohiro Osaki, Teruhisa Sakamoto, Soichiro Honjo, Keigo Ashida, Hiroaki Saito and Yoshiyuki Fujiwara Abstract Background: Treatment for tracheoesophageal fistula (TEF), a life-threatening complication after esophagectomy, is challenging. Case presentation: A 75-year-old man with thoracic esophageal cancer underwent subtotal esophagectomy and gastric tube reconstruction through the post-mediastinal root after neoadjuvant chemotherapy. Owing to postoperative anastomotic leakage, an abscess formed at the anastomotic region. Sustained inflammation from the abscess caused refractory TEF between the esophagogastric anastomotic site and membrane of the trachea, and several conservative therapies for TEF failed. Hence, the patient underwent surgery including division of the fistula, direct suturing of the leakage sites, and reinforcement with the flap of the thymus pedicle. As a result, the abscess and TEF disappeared after surgery and the patient was immediately administered an oral diet and discharged home 103 days after initial surgery. Conclusions: Although pedicle flaps for the reinforcement of TEF are usually obtained from muscle or pericardium, these flaps need enough lengths to overcome moving distance. We are the first in