Insecticide Mode of Action Technical Training Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Residential Pest Control Products Used in Inner City Communities in New York City
Journal of Exposure Science and Environmental Epidemiology (2010), 1–11 r 2010 Nature America, Inc. All rights reserved 1559-0631/10 www.nature.com/jes Characterization of residential pest control products used in inner city communities in New York City MEGAN K. HORTONa, J. BRYAN JACOBSONb, WENDY MCKELVEYb, DARRELL HOLMESa, BETTY FINCHERc, AUDREY QUANTANOc, BEINVENDIDA PAEZ DIAZc, FAYE SHABBAZZc, PEGGY SHEPARDc, ANDREW RUNDLEa AND ROBIN M. WHYATTa aColumbia Center for Children’s Environmental Health, Mailman School of Public Health, Columbia University, New York, New York, USA bNew York City Department of Health and Mental Hygiene, New York, New York, USA cWest Harlem Environmental Action, New York, New York, USA The Columbia Center for Children’s Environmental Health (CCCEH) previously reported widespread residential insecticide use in urban communities in New York City. Research suggests that pyrethroids are replacing organophosphates (OPs) in response to 2000–2001 US EPA pesticide regulations restricting OP use. A systematic assessment of active ingredients used for residential pest control is lacking. We queried a database of pesticide applications reported by licensed applicators between 1999 and 2005 and surveyed pest control products available in 145 stores within 29 zip codes in the CCCEH catchment area including Northern Manhattan and the South Bronx. Pyrethroids, pyrethrins, piperonyl butoxide, and hydramethylnon were the most common insecticide active ingredients reported as used by licensed pesticide applicators within the 29 zip codes of the CCCEH catchment area between 1999 and 2005. Use of certain pyrethroids and some non-spray insecticides such as fipronil and boric acid increased significantly by year (logistic regression, OR41.0, Po0.05), whereas use of OPs, including chlorpyrifos and diazinon decreased significantly by year (logistic regression, ORo1.0, Po0.05). -

Michigan Hop Management Guide 2018
2018 Michigan Hop Management Guide This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under Agreement No. 2015-09785. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. 2 Table of Contents Growth Stages………………………………………………………………………………………3 Weed Management………………………………………………………………………….4-5 Herbicides………………..……………………………………………………………………….6-7 Fungicides……………………………..………………………………………………………….8-9 Insecticides…………………………..………………………………………………………10-11 Miticides…………………………………………………………………………………………….12 Pesticide Toxicity to Beneficial Insects…………………………………………..13-14 Nutrient Management Considerations…………………………………………15-19 Scouting Calendar………………………………………………………………………………20 Information presented here does not supersede the label directions. To protect yourself, others, and the environment, always read the label before applying any pesticide. Although efforts have been made to check the accuracy of information presented, it is the responsibility of the person using this information to verify that it is correct by reading the corresponding pesticide label in its entirety before using the product. The information presented here is intended as a guide for Michigan hop growers in selecting pesticides and is for educational purposes only. Labels can and do change. For current label and MSDS information, visit one of the following free online databases: greenbook.net, cdms.com, and agrian.com The efficacies of products listed have not been evaluated on hop in Michigan. Reference to commercial products or trade names does not imply endorsement by Michigan State University Extension or bias against those not mentioned. This information was compiled by Erin Lizotte and Dr. Robert Sirrine with assistance from Dr. -

Identifying the Cause of Sediment Toxicity in Agricultural Sediments: the Role of Pyrethroids and Nine Seldom-Measured Hydrophobic Pesticides ⇑ Donald P
Chemosphere 90 (2013) 958–964 Contents lists available at SciVerse ScienceDirect Chemosphere journal homepage: www.elsevier.com/locate/chemosphere Identifying the cause of sediment toxicity in agricultural sediments: The role of pyrethroids and nine seldom-measured hydrophobic pesticides ⇑ Donald P. Weston a, , Yuping Ding b, Minghua Zhang c, Michael J. Lydy b a Department of Integrative Biology, University of California, 1005 Valley Life Sciences Bldg., Berkeley, CA 94720-3140, USA b Fisheries and Illinois Aquaculture Center and Department of Zoology, Southern Illinois University, 171 Life Sciences II, Carbondale, IL 62901, USA c Department of Land, Air, and Water Resources, University of California, Davis, CA 95616, USA highlights " Monitoring fails to test for many agricultural pesticides used in any given area. " Nine seldom-analyzed pesticides (e.g., abamectin) were tested for in sediments. " One-quarter of the sediment samples were toxic to the amphipod, Hyalella azteca. " The seldom-analyzed pesticides may have contributed to toxicity in a few samples. " Pyrethroid insecticides were responsible for the vast majority of toxicity. article info abstract Article history: Few currently used agricultural pesticides are routinely monitored for in the environment. Even if Received 10 January 2012 concentrations are known, sediment LC50 values are often lacking for common sediment toxicity testing Received in revised form 16 May 2012 species. To help fill this data gap, sediments in California’s Central Valley were tested for nine hydropho- Accepted 27 June 2012 bic pesticides seldom analyzed: abamectin, diazinon, dicofol, fenpropathrin, indoxacarb, methyl para- Available online 23 July 2012 thion, oxyfluorfen, propargite, and pyraclostrobin. Most were detected, but rarely at concentrations acutely toxic to Hyalella azteca or Chironomus dilutus. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Integrated Pest Management Plan 2021-22
Denair Unified School District INTEGRATED PEST MANAGEMENT PLAN Contacts Denair Unified School District 3460 Lester Rd., Denair, CA Mark Hodges (209) 632-7514 Ext 1215 [email protected] District IPM Coordinator Phone Number e-mail address IPM Statement It is the goal of Denair Unified School District to implement IPM by focusing on long-term prevention or suppression of pests through accurate pest identification, by frequent monitoring for pest presence, by applying appropriate action levels, and by making the habitat less conducive to pests using sanitation and mechanical and physical controls. Pesticides that are effective will be used in a manner that minimizes risks to people, property, and the environment, and only after other options have been shown ineffective. Pest Management Objectives: • Focus on long-term pest prevention using minimal pesticides. • Elimination of significant threats caused by pests to the health and safety of students, staff and the public. • Prevention of loss or damage to structures or property by pests. • Protection of environmental quality inside and outside buildings, in playgrounds and athletic areas, and throughout the Denair Unified School District facilities. IPM Team In addition to the IPM Coordinator, other individuals who are involved in purchasing, making IPM decisions, applying pesticides, and complying with the Healthy Schools Act requirements, include: Name Role Mark Hodges Making IPM Decisions Jerri Pierce Recordkeeping, and Making IPM Decisions Daniel Meza Applying Pesticides, Recordkeeping, -

Research/Investigación Effect of Dinotefuran
RESEARCH/INVESTIGACIÓN EFFECT OF DINOTEFURAN, INDOXACARB, AND IMIDACLOPRID ON SURVIVAL AND FITNESS OF TWO ARIZONA-NATIVE ENTOMOPATHOGENIC NEMATODES AGAINST HELICOVERPA ZEA (LEPIDOPTERA: NOCTUIDAE) P. D. Navarro, J. G. McMullen II, and S. P. Stock* University of Arizona, Department of Entomology, 1140 E South Campus Dr., Tucson, AZ 85721-0036. *Corresponding author: [email protected] ABSTRACT Navarro, P. D., J. G. McMullen II, and S. P. Stock. 2014. Effect of dinotefuran, indoxacarb, and imidacloprid on survival and fitness of two Arizona-native entomopathogenic nematodes against Helicoverpa zea (Lepidoptera: Noctuidae). Nematropica 44:64-73. The effect of three insecticides commonly used in Arizona, dinotefuran, indoxacarb, and imidacloprid, was evaluated on two Arizona-native entmopathogenic nematodes (EPN), Heterorhabditis sonorensis (Caborca strain) and Steinernema riobrave (SR-5 strain), using Helicoverpa zea (Lepidoptera: Noctuidae) as the insect host. Specifically, we assessed their effect on EPN survival and fitness (virulence and reproduction). Three application timings were considered: i) EPN applied first, insecticide applied 24 h later, ii) insecticide applied first, EPN applied 24 h later, and iii) simultaneous application of EPN and insecticide. Our results showed that infective juvenile (IJ) survival of S. riobrave and H. sonorensis was not significantly affected by the application of the selected insecticides. Indoxacarb had an ambiguous effect on the S. riobrave life cycle showing a synergistic effect in the virulence of this nematode but reducing its progeny production by two-fold. Similar results were observed for nematode progeny production when H. sonorensis and indoxacarb were applied simultaneously. All combinations of imidacloprid were antagonistic to the virulence of S. riobrave but additive with respect to the virulence of H. -

Biodegradation of Dicofol by Microbacterium Sp. D-2 Isolated
Lu et al. Appl Biol Chem (2019) 62:72 https://doi.org/10.1186/s13765-019-0480-y ARTICLE Open Access Biodegradation of dicofol by Microbacterium sp. D-2 isolated from pesticide-contaminated agricultural soil Peng Lu* , Hong‑ming Liu and Ai‑min Liu Abstract Dicofol is an organochlorine insecticide widely used to prevent pests worldwide. Consequently, serious environmen‑ tal problems have arisen from the application of dicofol. Bioremediation is an efective solution for dicofol persistence in the environment. In this study, a bacterial strain D‑2, identifed to genus Microbacterium, capable of degrading dicofol was isolated from dicofol‑contaminated agricultural soil. This represents the frst dicofol degrading bacterium isolated from this genus. Microbacterium sp. D‑2 degraded 50 mg/L dicofol within 24 h at a rate of 85.1%. Dicofol was dechlorinated by D‑2 and the further degradation metabolite was indentifed as p,p′‑dichlorobenzophenone(DCBP). Soils inoculated with Microbacterium sp. D‑2 degraded 81.9% of the dicofol, while soils without D‑2 only degraded 20.5% of the dicofol present. This fnding suggests that strain D‑2 has great potential in bioremediation of dicofol‑ contaminated soils. Keywords: Dicofol, Biodegradation, Microbacterium sp. D‑2, Bioremediation Introduction cause acute poising of fsh and shrimp, endocrine dis- Organochlorine pesticides (OCPs) are broad spectrum ruptive properties, and chronic toxicity to other organ- insecticides with high toxicity and long residual periods isms [10–13]. Terefore, it is believed that DCF would [1]. Tey have been widely used in the control of agricul- exert a negative infuence on both animals [14, 15] and tural pests, antisepsis in industrial production and the humans [16]. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Genetically Modified Baculoviruses for Pest
INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS This page intentionally left blank INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS EDITED BY LAWRENCE I. GILBERT SARJEET S. GILL Amsterdam • Boston • Heidelberg • London • New York • Oxford Paris • San Diego • San Francisco • Singapore • Sydney • Tokyo Academic Press is an imprint of Elsevier Academic Press, 32 Jamestown Road, London, NW1 7BU, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA ª 2010 Elsevier B.V. All rights reserved The chapters first appeared in Comprehensive Molecular Insect Science, edited by Lawrence I. Gilbert, Kostas Iatrou, and Sarjeet S. Gill (Elsevier, B.V. 2005). All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without permission in writing from the publishers. Permissions may be sought directly from Elsevier’s Rights Department in Oxford, UK: phone (þ44) 1865 843830, fax (þ44) 1865 853333, e-mail [email protected]. Requests may also be completed on-line via the homepage (http://www.elsevier.com/locate/permissions). Library of Congress Cataloging-in-Publication Data Insect control : biological and synthetic agents / editors-in-chief: Lawrence I. Gilbert, Sarjeet S. Gill. – 1st ed. p. cm. Includes bibliographical references and index. ISBN 978-0-12-381449-4 (alk. paper) 1. Insect pests–Control. 2. Insecticides. I. Gilbert, Lawrence I. (Lawrence Irwin), 1929- II. Gill, Sarjeet S. SB931.I42 2010 632’.7–dc22 2010010547 A catalogue record for this book is available from the British Library ISBN 978-0-12-381449-4 Cover Images: (Top Left) Important pest insect targeted by neonicotinoid insecticides: Sweet-potato whitefly, Bemisia tabaci; (Top Right) Control (bottom) and tebufenozide intoxicated by ingestion (top) larvae of the white tussock moth, from Chapter 4; (Bottom) Mode of action of Cry1A toxins, from Addendum A7. -

2.13 Fipronil Effect on the Frequency of Anomalous Brood in Honeybee Reared in Vitro Carina A.S
Hazards of pesticides to bees - 12th International Symposium of the ICP-PR Bee Protection Group, Ghent (Belgium), September 15-17, 2014 2.13 Fipronil effect on the frequency of anomalous brood in honeybee reared in vitro Carina A.S. Silva1, Elaine C.M. Silva-Zacarin2, Caio E.C. Domingues2, Fábio C. Abdalla2, Osmar Malaspina3, Roberta C.F. Nocelli1*. 1CCA - Centro de Ciências Agrarias, UFSCar - Universidade Federal de São Carlos. Rod. Anhanguera, SP 330, Km. 174, Araras – SP, Brasil. Email: [email protected]; Email: [email protected], *Phone: +55 (19) 3543- 2595 2LABEF – Laboratório de Biologia Estrutural e Funcional. Universidade Federal de São Carlos – UFSCar. Rodovia João Leme dos Santos (SP-264), Km. 110, Bairro Itinga, Sorocaba – SP, Brasil. 3CEIS – Centro de Estudos de Insetos Sociais. Universidade Estadual “Julio de Mesquita Filho” - UNESP. Av. 24 A, 1515, Bela Vista, Rio Claro – SP, Brasil. Abstract Larvae of honeybee workers were exposed to the insecticide fipronil during the feeding phase. To evaluate the effect of fipronil in the post-embryonic development of africanized Apis mellifera, bioassays of toxicity were done. The bioassays were performed by acute exposure applying 1μL of distilled water for control (I) and for experiments: 0.5 ng a.i./µL of fipronil; 5 ng a.i./µL of fipronil and 20 ng a.i./ µL of fipronil. Triplicates were performed for all treatments. The results showed that the rate of anomalous pupae in exposed honeybees was statistically significant in relationship to the control (p <0:03). The most frequent abnormalities were: high pigmentation on the proximal and distal larval body and body malformation, such as absence of head and limbs. -
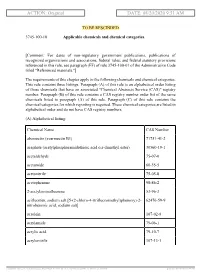
ACTION: Original DATE: 08/20/2020 9:51 AM
ACTION: Original DATE: 08/20/2020 9:51 AM TO BE RESCINDED 3745-100-10 Applicable chemicals and chemical categories. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations, federal rules, and federal statutory provisions referenced in this rule, see paragraph (FF) of rule 3745-100-01 of the Administrative Code titled "Referenced materials."] The requirements of this chapter apply to the following chemicals and chemical categories. This rule contains three listings. Paragraph (A) of this rule is an alphabetical order listing of those chemicals that have an associated "Chemical Abstracts Service (CAS)" registry number. Paragraph (B) of this rule contains a CAS registry number order list of the same chemicals listed in paragraph (A) of this rule. Paragraph (C) of this rule contains the chemical categories for which reporting is required. These chemical categories are listed in alphabetical order and do not have CAS registry numbers. (A) Alphabetical listing: Chemical Name CAS Number abamectin (avermectin B1) 71751-41-2 acephate (acetylphosphoramidothioic acid o,s-dimethyl ester) 30560-19-1 acetaldehyde 75-07-0 acetamide 60-35-5 acetonitrile 75-05-8 acetophenone 98-86-2 2-acetylaminofluorene 53-96-3 acifluorfen, sodium salt [5-(2-chloro-4-(trifluoromethyl)phenoxy)-2- 62476-59-9 nitrobenzoic acid, sodium salt] acrolein 107-02-8 acrylamide 79-06-1 acrylic acid 79-10-7 acrylonitrile 107-13-1 [ stylesheet: rule.xsl 2.14, authoring tool: RAS XMetaL R2_0F1, (dv: 0, p: 185720, pa: -

Western Flower Thrips Management on Greenhouse-Grown Crops
Western Flower Thrips Management on Greenhouse-Grown Crops Greenhouse producers worldwide are familiar with the Eggs hatch in two to four days. Nymphs feed on both western flower thrips, Frankliniella occidentalis (Pergande), leaves and flowers. The first nymphal stage lasts one to one of the most destructive insect pests of greenhouse- two days; the second nymphal stage, two to four days. grown crops. Western flower thrips, the primary thrips Second instar nymphs are typically more active and tend species encountered by greenhouse producers, is extremely to feed more than first instar nymphs. The second instar polyphagous, feeding on a wide-variety of horticultural nymph eventually migrates to the plant base and enters crops grown in both commercial and research greenhouses. the growing medium to pupate. Western flower thrips also This insect pest has been included in greenhouse pest pupate in leaf debris, on the plant, and in the open flowers control brochures since 1949. It was not considered a of certain types of plants including chrysanthemum. There major insect pest of greenhouse-grown crops until the are actually two “pupal” stages: a prepupa (or propupa) and 1980s. This publication addresses biology and damage; pupa. Both stages commonly occur in growing medium or scouting; and cultural, physical, insecticidal, and biological soil underneath benches. management. The issues discussed should provide insight Growing medium or soil type and pH and pupation depth on the importance of dealing with western flower thrips may influence pupal survival. Pupation depth depends on holistically instead of solely relying on insecticides. growing medium or soil type. Pupae stages do not feed Biology and Feeding Damage and are tolerant or immune to most insecticides commonly Knowledge of biology and damage is important in applied to manage western flower thrips nymphs and understanding the challenges associated with developing adults.