US5264213.Pdf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Genetically Modified Baculoviruses for Pest
INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS This page intentionally left blank INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS EDITED BY LAWRENCE I. GILBERT SARJEET S. GILL Amsterdam • Boston • Heidelberg • London • New York • Oxford Paris • San Diego • San Francisco • Singapore • Sydney • Tokyo Academic Press is an imprint of Elsevier Academic Press, 32 Jamestown Road, London, NW1 7BU, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA ª 2010 Elsevier B.V. All rights reserved The chapters first appeared in Comprehensive Molecular Insect Science, edited by Lawrence I. Gilbert, Kostas Iatrou, and Sarjeet S. Gill (Elsevier, B.V. 2005). All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without permission in writing from the publishers. Permissions may be sought directly from Elsevier’s Rights Department in Oxford, UK: phone (þ44) 1865 843830, fax (þ44) 1865 853333, e-mail [email protected]. Requests may also be completed on-line via the homepage (http://www.elsevier.com/locate/permissions). Library of Congress Cataloging-in-Publication Data Insect control : biological and synthetic agents / editors-in-chief: Lawrence I. Gilbert, Sarjeet S. Gill. – 1st ed. p. cm. Includes bibliographical references and index. ISBN 978-0-12-381449-4 (alk. paper) 1. Insect pests–Control. 2. Insecticides. I. Gilbert, Lawrence I. (Lawrence Irwin), 1929- II. Gill, Sarjeet S. SB931.I42 2010 632’.7–dc22 2010010547 A catalogue record for this book is available from the British Library ISBN 978-0-12-381449-4 Cover Images: (Top Left) Important pest insect targeted by neonicotinoid insecticides: Sweet-potato whitefly, Bemisia tabaci; (Top Right) Control (bottom) and tebufenozide intoxicated by ingestion (top) larvae of the white tussock moth, from Chapter 4; (Bottom) Mode of action of Cry1A toxins, from Addendum A7. -

Use Date Issued: September 2006 ______
United States Environmental Protection Agency Office of Prevention, Pesticides and Toxic Substances (7505P) _______________________________________________________ Pesticide Fact Sheet Name of Chemical: Metofluthrin Reason for Issuance: New Chemical Nonfood Use Date Issued: September 2006 _______________________________________________________ Description of Chemical IUPAC name: 2,3,5,6-tetrafluoro-4-(methoxymethyl)benzyl (EZ)- (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-prop-1- enylcyclopropanecarboxylate CAS name: [2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl 2,2-dimethyl-3-(1-propenyl)cyclopropanecarboxylate Common Name: Metofluthrin Empirical Formula: C18H20F4O3 EPA Chemical Code: 109709 Chemical Abstracts Service (CAS) Number: 240494-70-6 Chemical Class: Pyrethroid ester Registration Status: New Chemical, nonfood use Pesticide Type: Insecticide repellent not applied to human skin U.S.Technical Registrant : Sumitomo Chemical Company, LTD. 1330 Dillon Hghts. Ave. Baltimore, MD 21228 Use Pattern and Formulations Currently there are two end use products being proposed for metofluthrin. DeckMate ™ Mosquito Repellent Strip is an impregnated paper strip (~3,528 cm2) containing 1.82 percent metofluthrin as the active ingredient. The product also contains Bitrex ™ to discourage oral exposure to children or animals. The product is for use on patios, campsites, decks, cabanas, and other outdoor areas. One strip is applied per 10 ft × 10 ft outdoor area. Indoors the application rate is two strips per 50 m3. There are approximately 200 mg of metofluthrin initially in the strip. The strips can provide up to one week of protection Metofluthrin evaporates readily and therefore requires no external heat. Norm 1- is a personal outdoor insect repellent product consisting of a holder containing a replaceable cartridge insert coated with up to 50 mg of metofluthrin. -

Pesticide Resistance in Bed Bugs Everywhere!!!!!
2/24/2018 Pesticide Resistance in Bed bugs were virtually eradicated from the U.S. in Bed Bugs the post WWII era due to DDT and other powerful Shujuan (Lucy) Li insecticides. University of Arizona Alvaro Romero New Mexico State University 2 By the 1960s, bed bugs had developed resistance Public housing Apartments to DDT, methoxychlor and analogues, BHC, Schools dieldrin and analogues , and pyrethrins ( Busvine 1958, Hospitals Nursing homes Cwilich & Mer 1957, Mallis and Miller 1964 ) . Homes Transportation Child care Medical facilities Hotels & motels Health care facilities Airports Movie theaters Department stores Products, vendors, or commercial services mentioned or pictured in this seminar are for Everywhere!!!!! illustrative purposes only and are not meant to be endorsements. 3 4 University of Arizona; Arizona Pest Management Center 1 2/24/2018 Possible reasons for treatment failure? Missed some Clutter Reintroduction Have you seen these after treatments? 5 6 Dose - response assays for field - collected strains Bed bugs survived direct insecticide sprays 99 deltamethrin 90 Ft. Dix F1 50 ) e l a c 10 s t CIN1 i b o 1.0 r p ( y t i l a t r 99 - cyhalothrin o m e 90 g a t n Resistance ratio (RR) at least 6,000 !!! e c Ft. Dix r 50 e P 10 CIN1 Suspend® ( Deltamethrin ) 1.0 10 -7 10 -6 10 -5 10 -4 10 -3 10 -2 10 -1 10 0 10 1 10 2 10 3 10 4 Treatment (mg active ingredient/cm 2 ) Products, vendors, or commercial services mentioned or pictured in this seminar are for illustrative purposes only and are not meant Romero et al. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Application of ACQUITY TQD for the Analysis of Pesticide Residues
[ application application note note ] ] APPLICATION OF ACQUIT Y TQD FOR THE ANALYSIS OF PESTICIDE RESIduES IN Baby FOOD James Morphet and Peter Hancock Waters Corporation, Manchester, UK OVERVIEW This application note assesses the suitability of the Waters® ACQUITY™ TQD for tandem quadrupole-based analysis of pesticide residue in baby food. Polarity switching, differing dwell times and ion ratio robustness will also be assessed. INTRODUCTION The European Union residue monitoring program, 2005-2007, establishes the need to cover 55 active ingredients in various foods, Figure 1. The Waters ACQUITY TQD with the TQ Detector. including baby foods1, 2. Twenty of these pesticides are suitable for multi-residue LC/MS analysis; only one has a negative polarity in electrospray mode, normally requiring two injections (one in each EXPERIMENTAL polarity ion mode). Consequently compounds with negative polarity 3 are often excluded from monitoring programs. Ideally, these should The sample extraction method has been previously reported . be determined in a single analysis with polarity switching. Extracts of blank baby food matrix in acetonitrile were provided along with mixtures of the compounds in acetonitrile by the Central Chemists analyzing pesticide residues are under increasing pres- Science Laboratory (CSL), York, UK. Extracts for injection were sure to broaden the range of pesticides determined in a single prepared by spiking the compounds into the baby food matrix. The analysis: to improve limits of detection, precision and quantitation; supernatant was analyzed on the ACQUITY TQD following dilution to increase confidence in the validity of residue data; to provide with water (1:9) v/v. faster methods and to reduce usage of hazardous solvents while maintaining or reducing costs. -

Fate of Pyriproxyfen in Soils and Plants
toxics Review Fate of Pyriproxyfen in Soils and Plants James Devillers CTIS, 3 Chemin de la Gravière, 69140 Rillieux-La-Pape, France; [email protected] Received: 17 February 2020; Accepted: 10 March 2020; Published: 13 March 2020 Abstract: Since the 1990s, the insect growth regulator pyriproxyfen has been widely used worldwide as a larvicide in vector control and in agriculture to fight a very large number of pests. Due to its widespread use it is of first importance to know how pyriproxyfen behaves in the terrestrial ecosystems. This was the goal of this work to establish the fate profile of pyriproxyfen in soils and plants. Thus, in soil, pyriproxyfen photodegrades slowly but its aerobic degradation is fast. The insecticide presents a high tendency to adsorb onto soils and it is not subject to leaching into groundwater. On the contrary its two main metabolites (40-OH-Pyr and PYPAC) show a different fate in soil. When sprayed to plants, pyriproxyfen behaves as a translaminar insecticide. Its half-life in plants ranges from less than one week to about three weeks. The review ends by showing how the fate profile of pyriproxyfen in soils and plants influences the adverse effects of the molecule on non-target organisms. Keywords: pyriproxyfen; soil; plant; metabolites; insect growth regulator; endocrine disruptor; terrestrial ecosystems 1. Introduction The behavior of pesticides in soils is under the dependence of physical, chemical, and biological processes including volatilization, sorption–desorption, aerobic and anaerobic degradation, uptake and release by plants, run-off, and leaching. These various complex processes control the transport of pesticides within the soil and their transfer from the soil to the other environmental compartments [1–3]. -

Free of Analytes Matrixes
FREE OF ANALYTES MATRIXES Matrix: Green beans Description: Green beans in plastic container sealed with pressure lid and screw cap. Approximated weight: 50g Date of analysis: May 2019 Analyzed analytes: Pesticides residues (See annex). Results: There was no presence of any of the target analytes (See annex) above the quantification limit. NOTES The material has been kept in temperature-controlled freezer and is sent frozen. Upon receive store in freezer until analysis. The test material has been analyzed by the TestQual’s collaborator laboratory accredited for the analysis of the annexed pesticides, therefore ensuring the lack of pesticides in concentrations higher than the limit of quantification of the laboratory: 10µg/Kg The list of the analyzed pesticides is the one TestQual works with regularly in our proficiency test of pesticides residues. You can check the full list of analytes in the protocol or request the documentation using the contact data on our website: http://testqual.com/contacto/?lng=en Page 1 / 3 ANNEX 2-Phenylphenol Carbendazim Dichlormid Fenazaquin 3,5-Dichloroaniline Carbophenothion Dichlobenil Fenbuconazole 3-Hydroxy-carbofuran Carbofuran Diclobutrazol Fenbutatin oxide 4,4-Dichlorobenzophenone Chlorantraniliprole Dichlofluanid Fenchlorphos Abamectin Chlorbromuron Diclofop-methyl Fenhexamid Acephate Chlorfenapyr Dicloran Fenitrothion Acetamiprid Chlorfenvinphos Dicrotophos Fenoxycarb Acetochlor Chlormephos Dieldrin Fenpropathrin Aclonifen Chloroneb Diethofencarb Fenpropimorph Acrinathrin Chloropropylate Difenoconazole -

1 of 3 GC+LC-USA
Updated: 07/18/2016 1 of 3 GC+LC-USA Limit of Quantitation (LOQ): 0.010 mg/kg (ppm) Sample Types: Low Fat Content Samples Minimum Sample Size: 100 grams (~1/4 pound). Certain products require more for better sample representation. Instrument: GC-MS/MS and LC-MS/MS Turnaround: 24-48 hours Accreditation: Part of AGQ USA's ISO/IEC 17025 Accreditation Scope 4,4'-Dichlorobenzophenone Bupirimate Cyantraniliprole Diflufenican Abamectin Buprofezin Cyazofamid Dimethoate Acephate Butachlor Cycloate Dimethoate (Sum) Acequinocyl Butocarboxim Cycloxydim Dimethomorph Acetamiprid Butralin Cyflufenamid Diniconazole Acetochlor Cadusafos Cyfluthrin Dinocap Acrinathrin Captafol Cymoxanil Dinotefuran Alachlor Captan Cyproconazole Diphenylamine Aldicarb Captan (Sum) Cyprodinil Disulfoton Aldicarb (Sum) Carbaryl Cyromazine Disulfoton (Sum) Aldicarb-sulfone Carbofuran DDD-o,p Disulfoton-sulfone Aldicarb-sulfoxide Carbofuran-3-hydroxy DDD-p,p +DDT-o,p Disulfoton-sulfoxide Aldrin Carbophenothion DDE-o,p Ditalimfos Ametryn Carbosulfan DDE-p,p Diuron Amitraz Carboxine DDT (Sum) Dodemorph Atrazine Carfentrazone-ethyl DDT-p,p Dodine Azadirachtin Chinomethionat DEET Emamectin Benzoate Azamethiphos Chlorantraniliprole Deltamethrin Endosulfan (A+B+Sulf) Azinphos-ethyl Chlordane Demeton Endosulfan Alfa Azinphos-methyl Chlordane Trans Demeton-S-methyl-sulfone Endosulfan Beta Azoxystrobin Chlorfenapyr Desmedipham Endosulfan Sulfate Benalaxyl Chlorfenson Diafenturion Endrin Ben-Carb-TPM (Sum) Chlorfenvinphos Dialifos EPN Bendiocarb Chlorfluazuron Diazinon Epoxiconazole -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -

Report 27 Quantitative Determination of Pesticide
EURL for Cereals and Feeding stuff National Food Institute Technical University of Denmark Validation Report 27 Determination of pesticide residues in fish feed by GC-MS/MS (modified QuEChERS method) Mette Erecius Poulsen Susan Strange Herrmann December 2017 Page 2 of 26 CONTENT: 1. Introduction ...................................................................................................................................... 3 2. Principle of analysis......................................................................................................................... 3 3. Validation design ............................................................................................................................. 4 4. Chromatograms and calibration curves .......................................................................................... 4 5. Validation parameters...................................................................................................................... 4 6. Criteria for the acceptance of validation results ............................................................................. 5 7. Results and conclusion ..................................................................................................................... 5 9. References ........................................................................................................................................ 6 Appendix 1. MRM transitions for GC-MS/MS. ................................................................................... -
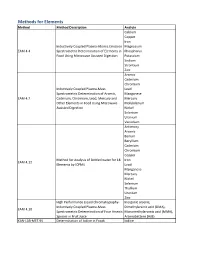
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and