(AIOM) 25-27 October, 2019 - Rome, Italy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sustainability Reporting 2011
The Hera Group Sustainability Report for 2011 contains figures for the three areas of responsibility: economic, social and environmental. Focus on commitments made, the results obtained and the outlook for the future. Contents Letter to stakeholders................................................................................................................................................ 4 The Report .................................................................................................................................................. 5 Reading this Report .................................................................................................................................................. 5 Drawing up this report .............................................................................................................................................. 7 About us .................................................................................................................................................... 11 Hera today............................................................................................................................................................... 11 History .................................................................................................................................................................... 11 Services managed .................................................................................................................................................. -

Politecnico Di Torino
POLITECNICO DI TORINO Department of Mechanical and Aerospace Engineering Master of Science in Automotive Engineering Master of Science Thesis Business Development in Medium-Sized Company Supervisor Prof. Paolo Ferrero Co-supervisor Candidate Linda Negro Shenouda Fraig Academic Year 2019/2020 Acknowledgment I would like to express my deepest gratitude and thankfulness for my family for their continuous support and motivation throughout the period of my master’s degree. I would like also to express special thanks to Professor Paolo Ferrero who gave me the chance to do this thesis under his supervision and guidance and giving me an opportunity to join the company “Spesso Gasket” for my internship. I am grateful for the team of “Spesso Gasket”, Ms. Linda my direct supervisor and Spesso’s sales manager for her support and highly-appreciated contribution to my work, to Ms. Alessandra and Ms. Viviana for the welcoming and friendly working environment that they provided throughout the internship. It was an experience I will never forget. Abstract In the modern-day working environment, companies should thrive daily to innovate and outgrow their competition, or else risk losing their businesses. This is even more relevant for small/medium enterprises (SMEs) were the consequences could be more severe. There are two main objectives for this dissertation. The first one is to develop a comprehensive view of the concept of business development, especially in the case of SMEs. This part of the thesis is mainly based on researching the available academic publications, extracting the important and relevant information and combining all the data to form a well-structured idea of the following question “What is business development?”. -

Enzo Ferrari
30Dipartimento di Ingegneria Department“Enzo of Ferrari” Engineering “Enzo Ferrari” University of Emilia-Romagna (MUNER), a synergistic 2020 marks 30 years of association among many Universities, Emilia-Romagna Region, Engineering in Modena. The “Enzo Ferrari” and automotive industries that represent worldwide the Engineering Department (DIEF), which was excellence of the “Made in Italy” automotive that is rooted in so called after the faculty of the same name, the same territory in which DIEF is present. is an active part of UNIMORE’s Engineering School and is comprised of 110 professors and researchers, besides more than 5000 Therefore, in recent years the teaching portfolio expanded students. The teaching portfolio includes 7 by including new international and interuniversity Master Bachelor courses, 10 Master courses, courses in automotive engineering, which represent one of and 3 Ph.D. schools, covering all the most DIEF’s main hallmarks. Lecture courses therefore benefit from ever relevant and cutting-edge topics in engineering: renewed contents that trigger and motivate students, giving them from mechanics to automotive, from materials a long-lasting and robust higher education, highly valued in to electronics, from computer science to civil the job market. engineering, from telecommunications to environment. A significant contribution to applied research comes from Interdepartmental Centres, specifically those housed in By acknowledging the solid intertwining DIEF such as AIRI (Artificial Intelligence Research and Innovation between -

N° 13 – Zapping – 2007
Speciale Speciale Immobiliare Immobiliare OVADA LIGURIA pag. 48 pag. 49 Registrazione Tribunale di Alessandria n° 552 del 31/01/03 - Direttore Responsabile Raimondo Bovone - Editore: Publitre S.r.l. - S IL GIORNALE QUINDICINALEVia GRATUITO G. Ferraris, 19 - ALESSANDRIA CON LE - Info: MIGLIORI OCCASIONI E INIZIATIVE COMMERCIALI DELLA TUA CITTA’ Tel. 0131.260434 - Fax 0131.257630 N°13 • DAL 02/07 AL 15/07/2007 • ALESSANDRIA E PROVINCIA • VOGHERA E VERCELLI - E-Mail: [email protected]: TATI BOOK (TO) 2 www.zappingonline.net www.zappingonline.net www.zappingonline.net www.zappingonline.net @ CAPRA da latte 16 BOULEDOGUE francese IL 10/04/07 sono nati 6 REGALO cagnolina di 2 @ 12 BELLISSIMI gattini @ CUCCIOLI di mesi primipara pura cucciola molto bella , al- cuccioli di rottweiller da anni bianca pelo corto, Europei a pelo corto e CHIHUAHUA , vaccinati razza Saanen, registrata tissima genealogia, otti- genitori di grosso equili- abituata a stare in ap- semi-lungo, maschi e e sverminati. Genitori vi- ASL con marchio aurico- ma salute nata in casa, brio (importantissimo partamento tel. 0131 femmine, tutti i colori sibili.vendo a ottimo lare, esente brucellosi. carattere eccezionale per avere cani sicuri) 585310 (rosso, champagne, gri- prezzo tel ore pasti al n. @ ABBIGLIAMENTO uo- Vendo. Tel. 329 vendo. tel. 338 con vaccino tre svermi- REGALO gattini 2 maschi gio tigrato, marrone/ne- 0131 346019 o 320 mo nuovo e usato e ac- 4550927 9245028 nature microchip e pede- neri, una femmina nera, ro), carini, dolci, nati 1106516 cessori: cravatte, cintu- @ CERCO coppia car- CANI di razza bulldog gree salo amanti della un maschio grigio certosi- presumibilmente ai primi @ CUCCIOLI di NORVE- re.. -

A Fountain for Memory
1 A FOUNTAIN FOR MEMORY: The Trevi Flow of Power and Transcultural Performance Pam Krist, PhD Thesis School of Modern Languages, Literature and Culture Royal Holloway, University of London 2015 2 3 Abstract In memory studies much research on monuments focuses on those with traumatic or controversial associations whilst others can be overlooked. The thesis explores this gap and seeks to supplement the critical understanding of a populist monument as a nexus for cultural remembering. The Trevi Fountain in Rome is chosen because it is a conduit for the flow of multivalent imagery, ideological manipulation, and ever-evolving performances of memory, from design plans to mediated representations. The thesis begins by locating the historical pre-material and material presences of the Fountain, establishing this contextual consideration as contributory to memory studies. It then surveys the field of theory to build a necessarily flexible conceptual framework for researching the Fountain which, given the movement and sound of water and the coin-throwing ritual, differs from a static monument in its memorial connotations. The interpretations of the illusory Trevi design and its myths are explored before employing a cross-disciplinary approach to the intertextuality of its presences and its performative potential in art, literature, film, music, advertising and on the Internet. The thesis concludes with questions about the digital Trevi and dilution of memory. Gathering strength throughout is the premise of the Fountain as a transcultural vehicle for dominant ideologies ‒ from the papal to commercial, the Grand Tour to cyber tourism ‒ seeking to control remembering and forgetting. Sometimes these are undermined by the social and inventive practices of memory. -

Annual Report 2019 Annual Report 2019
ANNUAL REPORT 2019 ANNUAL REPORT 2019 FAI – Fondo Ambiente Italiano Headquarters La Cavallerizza - Via Carlo Foldi, 2 - 20135 Milan, Italy Tel. (+39) 02 4676151 - email: [email protected] Rome Office Via delle Botteghe Oscure, 32 - 00186 Rome, Italy Tel. (+39) 06 689675 2 - email: [email protected] www.fondoambiente.it FAI - THE NATIONAL OUR VISION TRUST FOR ITALY FAI in 2023 FAI – Fondo Ambiente Italiano (the ■ The main focus of FAI’s work is to look after the special National Trust for Italy) is a non-profit places it has either received through donations or bequests foundation that operates for the and/or manages under concession. These places are viewed safeguarding of Italy’s natural and artistic and treated as fulcrums within the landscape and social/ heritage. cultural/economic systems in which they are located. FAI has always drawn inspiration from ■ Today, FAI’s monuments and landscapes are located for the National Trust for England, Wales the most part in the north of Italy. We want these to grow in & Northern Ireland. It is a member of variety and number, across all of the regions of Italy, with INTO – the International National Trusts a special focus on Rome. It is crucial that we are able to Organization – and is recognized as a guarantee their sustainability. legal entity through Italian Presidential Decree No. 941 of 3/12/1975). ■ FAI intends to increase its commitment to building on the relationship between the places in its care and the people Since its foundation in 1975, FAI has and families who enjoy them, in order to respond to their been promoting a culture of respect for various needs and desires. -

Sustainability Report 2009 Was Drawn up on the Basis of the AA1000 Standard Which Provides the Steps Required for Preparing Social and Sustainability Reports
Contents Letter from the Chairman of the Board..................................................................................................................... 4 Letter from the Chief Executive Officer ................................................................................................................... 5 The Report .................................................................................................................................................. 6 Reading this Report .................................................................................................................................................. 6 Drawing up this report .............................................................................................................................................. 8 About us .................................................................................................................................................... 11 Hera today............................................................................................................................................................... 11 History .................................................................................................................................................................... 11 Services managed ................................................................................................................................................... 12 Mission and Values................................................................................................................................................ -

Peugeot 50Cc Scooter Cranks T-Max Malossi Power
October 2010 E & OE 2010 PEUGEOT 50CC SCOOTER CRANKS Replacing your Peugeot 50cc scooter's crankshaft used to be a costly affair. The only source of the later cam driven crank would be directly from a franchised main dealer. Well, thanks to VE (UK), a replacement unit can now be purchased for a much more reasonable sum of money. With both the early and late versions of the crank now available, for scooters with or without the air feed pipe, savings can be made on both fronts. The VE cranks are top quality both in materials used and performance. These high quality cranks produced by Top Racing fit a multitude of models of Peugeot 50cc scooters including: Buxy, Elyseo, Looxor, Metal X, Speedake, Speedfight, Squab, Trekker, Vivacity and Zenith. VB20431 STANDARD CRANKSHAFT - FOR SCOOTERS WITH AIR FEED PIPE VB20051 STANDARD CRANKSHAFT - FOR SCOOTERS WITHOUT AIR FEED PIPE T-MAX MALOSSI POWER Malossi have developed the following range of items for the Yamaha T-Max for use in their very successful race series in Italy, and have developed them over a considerable amount of race re- search and development. Malossi have pulled out all the stops once again and produced a 560cc cylinder kit for the Yamaha T-Max 500. The single block alloy twin cylinder is produced from a special blend of alloys that is hardened and stress relieved and machined to extremely tight tolerances that you have come to expect from all Malossi products. Malossi have worked their magic to produce a product that will see an increase of approximately 15% increase in overall power. -
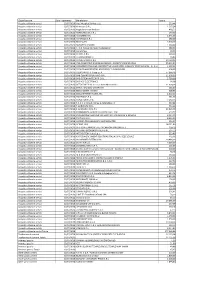
Pagamenti III Trimestre 2018.Pdf
Classificazione Data Pagamento Beneficiario Totale Acquisto di beni e servizi 02/07/2018 Aiesi Hospital Service s.a.s. 293,44 Acquisto di beni e servizi 02/07/2018 Airmaster S.R.L 1.567,24 Acquisto di beni e servizi 02/07/2018 Anglo American Book co 438,52 Acquisto di beni e servizi 02/07/2018 ARANCHO DOC S.R.L. 240,00 Acquisto di beni e servizi 02/07/2018 AS SISTEMI SRL 2.545,00 Acquisto di beni e servizi 02/07/2018 ASTERISCO S.R.L. 180,00 Acquisto di beni e servizi 02/07/2018 BLUSEA SRL 5.000,00 Acquisto di beni e servizi 02/07/2018 BORGHESI SIMONA 1.560,00 Acquisto di beni e servizi 02/07/2018 C. N.N. Consorzio Nuovi Noleggiatori 480,00 Acquisto di beni e servizi 02/07/2018 CartaSi Spa 18,33 Acquisto di beni e servizi 02/07/2018 CHEMIL S.R.L. 219,10 Acquisto di beni e servizi 02/07/2018 CHIAVARI MARCO 24,80 Acquisto di beni e servizi 02/07/2018 CLIMATECNICA S.R.L. 19.710,00 Acquisto di beni e servizi 02/07/2018 CNS-CONSORZIO NAZIONALE SERVIZI - SOCIETA' COOPERATIVA 29.604,24 Acquisto di beni e servizi 02/07/2018 CONSORZIO ITALIANO COOPERATIVE LAVORATORI AUSILIARI TRAFFICO SCRL - C.I.C.L.A.T. 1.507,22 Acquisto di beni e servizi 02/07/2018 COOP.TRASPORTATORI ARGELATO E S.GIORGIO SRL 300,00 Acquisto di beni e servizi 02/07/2018 COOPSERVICE S. Coop. p. A. 37.066,26 Acquisto di beni e servizi 02/07/2018 DAB CENTRO OPERATIVO S.R.L. -

Repubblica Italiana
REPUBBLICA ITALIANA BOLLETTINO UFFICIALE DIREZIONE E REDAZIONE PRESSO LA PRESIDENZA DELLA REGIONE - VIALE ALDO MORO 52 - BOLOGNA Parte seconda - N. 283 Anno 44 28 novembre 2013 N. 353 DELIBERAZIONE DELLA GIUNTA REGIONALE 18 NOVEMBRE 2013, N. 1670 Integrazione alla “Raccolta aggiornata delle disposizioni regionali per l’attuazione degli ammortizzatori sociali in deroga” di cui alla DGR 261/2013 - 2° provvedimento 2 DELIBERAZIONE DELLA GIUNTA REGIONALE 18 NOVEMBRE 2013, N. 1671 Concessione degli ammortizzatori sociali in deroga. Quarto provvedimento di autorizzazione 3 2 28-11-2013 - BOLLETTINO UFFICIALE DELLA REGIONE EMILIA-ROMAGNA - PARTE SECONDA - N. 353 REGIONE EMILIA-ROMAGNA autorizzate solo sulla base dell’accordo sindacale stipulato tra le parti in sede aziendale o territoriale senza la necessità che DELIBERAZIONE DELLA GIUNTA REGIONALE 18 NO- sia svolto un ulteriore esame”. VEMBRE 2013, N. 1670 Dato atto, inoltre, che nell’incontro del 28/10/2013 del “Ta- Integrazione alla "Raccolta aggiornata delle disposizio- volo tecnico di monitoraggio ai sensi della propria deliberazione ni regionali per l'attuazione degli ammortizzatori sociali in n. 692/09” di cui al Decreto Assessorile n. 3 del 8/6/2010, la deroga" di cui alla DGR 261/2013 - 2° provvedimento Direzione regionale INPS ha comunicato che l’’incentivo all’au- toimprenditorialità istituito con l’art. 7ter comma 7 del D.L. LA GIUNTA DELLA REGIONE EMILIA-ROMAGNA n. 5/2009 non è stata prorogato nuovamente per l’anno 2013 ri- Richiamata l’Intesa tra Regione Emilia-Romagna e Parti so- tenendo, pertanto, che sulle stesse basi non possa più trovare ciali per l’accesso agli ammortizzatori sociali in deroga anno applicazione neppure la disposizione regionale di cui al punto 6. -

Multidisciplinarity and Innovation ASP Projects Multidisciplinarity and Innovation ASP Projects 8 Preface
ALTA SCUOLA POLITECNICA Multidisciplinarity and innovation ASP projects Multidisciplinarity and innovation ASP projects 8 Preface This book marks the completion of the course of studies for the eighth cycle of students of the Alta Scuola Politecnica. Created in 2004, the Alta Scuola Politecnica programme draws on the experience of the Politec- nico di Milano and the Politecnico di Torino, two universities with different histories, conditions and methods, but sharing the desire to offer a highly-innovative course of studies to selected talented students with an interest on multi-disciplinarity. From the outset, this ambitious project aimed to create an axis of learning between Turin and Milan. Today, as we witness the growing economic and social bonds between these two cities – both of which are key to the Italian economy – we are increasingly confident that we made the right decision ten years ago, a decision that during this time has come to fruition and has evolved and improved. The world is witnessing a very fast technological and social development that is leading to the emergence of new paradigms; therefore, technical professionals of the future should not only be specialists in a given discipline, but also capable of building innovative solutions that are most suit- able to be transferred to the products and services of the future. At the same time, when dealing with particularly talented students, we believe that universities should do more than simply issue degrees - they should also prepare these students to become future leaders and meet the specific demands that prospective employers cast on this particular segment of graduates. -

Adobe Photoshop
SESSANTUN’ANNI DI INESAURIBILE EDITORIALE GIOVINEZZA il Club Mille Miglia, chiude il suo nostra gara, difficilmente riscontrabile in eventi sessantunesimo anno di vita più attivo che mai, similari, è stato degno del blasone di questa con molte soddisfazioni e qualche rimpianto. competizione, della quale abbiamo celebrato i A renderci orgogliosi del nostro Club è la settant’anni. vitalità che seguita a dimostrare, grazie alla Il livello qualitativo della corsa è stato raggiunto partecipazione e all’entusiasmo dei Soci, in solo grazie alla generosità delle aziende che particolare di alcuni Fondatori. ci hanno sostenuto economicamente: a loro Il rammarico scaturisce dall’aver perduto alcuni siamo doppiamente riconoscenti perché - pur carissimi amici, storici protagonisti del nostro soddisfatti per aver garantito la visibilità e il Club: dopo il nostro indimenticabile Segretario ritorno d’immagine promessi - è evidente che Generale - Raoul Patrizi - e un Vicepresidente queste sponsorizzazioni nascono prima dalla - il grandissimo Gino Munaron - ci ha lasciato passione che dal calcolo economico. anche Andrea Curami. Oltre alla Coppa Mazzotti, integralmente Di Gino e di Andrea troverete un ricordo organizzata dal Club, abbiamo fornito nelle prossime pagine ma, come per Raoul, patrocinio e supporto logistico a un’altra avvertiremo costantemente la loro assenza: la competizione che ha ottenuto un lusinghiero loro passione e la loro competenza avrebbero successo: il Rally 1000 Miglia Storico, abbinato ulteriormente accresciuto il successo delle al Memorial Busseni, del quale parleremo sulla nostre iniziative. Freccia Rossa successiva. Essendo un convinto assertore del motto “chi Il prossimo numero della nostra rivista seguirà si loda si imbroda”, non avrei mai utilizzato il a breve quello che state leggendo, essendo termine successo, se lo stesso non fosse stato numerosi i temi da trattare in chiusura d’anno.