Direct and Indirect Influences of Intercrops on the Coconut Defoliator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Old World Bollworm Management Program Puerto Rico Environmental Assessment, September 2015
United States Department of Agriculture Old World Bollworm Marketing and Regulatory Management Program Programs Animal and Plant Health Inspection Service Puerto Rico Environmental Assessment September 2015 Old World Bollworm Managment Program Puerto Rico Environmental Assessment September 2015 Agency Contact: Eileen Smith Pest Detection and Emergency Programs Plant Protection and Quarantine Animal and Plant Health Inspection Service U.S. Department of Agriculture 4700 River Road, Unit 134 Riverdale, MD 20737 Non-Discrimination Policy The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) To File an Employment Complaint If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. Additional information can be found online at http://www.ascr.usda.gov/complaint_filing_file.html. To File a Program Complaint If you wish to file a Civil Rights program complaint of discrimination, complete the USDA Program Discrimination Complaint Form (PDF), found online at http://www.ascr.usda.gov/complaint_filing_cust.html, or at any USDA office, or call (866) 632-9992 to request the form. -

Page 1 Jpn. J. Environ. Entomol. Zool. 19 (2) : 59-67 (2008) 19 2 : 59-67
]pn. ]pn. ]. Environ. Entoillo l. Zoo l. 19 (2) : 59 - 67 (2008) 環動昆第19 巻第2号: 59 - 67 (2008) 。riginal Article Comparisons of cocoon density and survival processes of the blue 幽 striped nettle grub moth Parasa lepida (Cramer) between deciduous and evergreen trees Hiroichi Hiroichi Sawada 1) ,Y oshihisa Masumoto 1), Takashi Matsumoto 2) and Takayoshi Nishida 3) 1) 1) School of Environmental Science ,The University of Shiga Prefecture ,日 ikone , Shiga 522-8533 , Japan 2) 2) Graduate School of Human and Environmental Studies ,Kyoto University ,Kyoto 606-850 1. Japan 3) 3) Laboratory of Insect Ecology , Graduate School of Agriculture ,Kyoto University ,Kyoto 606-8502 , Japan (Received (Received March 17 , 2008 ; Accepted May 1, 2008) Abstract We studied population dynamics of the blue-striped nettle grub moth Parasa lepida (Cramer) , in terms of cocoon density density over four years from 2004 to 2007 at the campus of The University of Shiga Prefecture ,Hikone , western Japan Japan on a wide range of host trees including both deciduous trees (36 spp. of 282 individual trees) and evergreen trees trees (15 spp. of 122 individual trees). Detailed survival processes were examined by tracking developmental stages both both on the deciduous Chinese tallow tree Triadica seb 俳ra (1.) Small (Euphorbiaceae) and an evergreen oak Quercus myrsinaefolia myrsinaefolia Blume (Fagaceae) to identify factors responsible for the population dynamics and the host utilization patterns. patterns. The density of cocoons was significantly higher in deciduous hosts than in evergreen hosts in the first generation , but this tendency disappeared in the second generation. Life table analyses revealed there was a higher cocoon cocoon density in deciduous T. -

Current Status of Bio-Pesticides Development, Farmer's Acceptance
CURRENT STATUS OF BIO-PESTICIDES DEVELOPMENT, FARMER’S ACCEPTANCE AND UTILIZATION, AND FUTURE PERSPECTIVE IN TAIWAN Suey-Sheng Kao Biopesticides Division Taiwan Agricultural Chemicals and Toxic Substances Research Institute Council of Agriculture, Wufeng, Taichung, 413 Taiwan ROC ABSTRACT Taiwan has made significant progress in the development and application of bio-pesticides and microbial pesticides in the past two decades. Sex pheromones of Cylas formicarius elegantulua, Eucosma notanthes, Spodoptera litura, and S. exgiua have been synthesized, formulated, and utilized in many ways with satisfactory results, including monitoring, mass trapping, and mating disruption. Nomurea riley was found to be pathogenic to Heliocoverpa armigera and Spodoptera exigua. It effectively controlled H. armigera during field applications. Metarhizium anisopliae was applied to control S. exigua, Brontispa longissima, Loadelphax striatellus. M. anisopliae was also used for destruxin production. Destruxins produced showed high virulence to S. exigua. Beauveria bassiana in soil was found to be lethal to Cylas formicarius. B. bassiana preparations were effective in controlling C. formicarius, Ostrinia furnacalis, and Riptorus lineasis. B. bassiana was also pathogenic to Lipaphis erysimi. The optimal growth and maximum sporulation condition of three isolates of Verticillium lecanii were investigated as well. V. lecanii was reported to be highly pathogenic to Myzus persicae, Macrosiphoniella sanborni, Toxoptera aurantii, Liaphis erysimi, Aphis gossypii, and Saissetia oleae. Many aspects relating to these fungi, e.g., characterization, breeding fungicide-resistant mutants, production process, recovery, formulation, factors affecting infectivity, and compatibility with pesticides, were also evaluated. Granulosis viruses of Plutella xylostella, Artogeia rapae, and nuclear polyhedrosis viruses of Spodoptera litura, and S. exigua were identified and field tested against their own hosts. -

Assessment of Risks and Potential of Injection Techniques in Integrated Programs to Eradicate the Red Palm Weevil: Review and New Perspectives
Review Assessment of risks and potential of injection techniques in integrated programs to eradicate the red palm weevil: review and new perspectives Michel FERRY*, Susi GOMEZ INRA, Estación Phoenix, Assessment of risks and potential of injection techniques in integrated programs to Apartado 996, 03201 Elche, eradicate the red palm weevil: review and new perspectives. Spain, Abstract – Introduction. Plants develop mechanisms that allow them to compartmentalize injuries [email protected] that they suffer during their life. In trees, pruning and injection treatments must be used in accordance with precise rules to reduce risks resulting from the injuries created. Sealing in palms. Palms, con- trary to widespread belief, are quite capable of “healing” injuries (sealing); because of an anatomy quite different from trees, the sealing process in palms is much simpler. Compartmentalization of injection wounds. The controversy on the use of injection in trees is due essentially to initial mis- takes that have then been rectified. Injection in palms against the red palm weevil. For palms, for decades, this technique has been employed without problems and with great efficiency against various pests, including Rhynchophorus ferrugineus, the red palm weevil (RPW). Its use has been reserved for exceptional situations either to face abnormal pest proliferation, uncontrollable by other techniques, or to implement eradication programs. Integrated eradication program. In such a pro- gram, the main aim of injection treatments is preventive. With long-persistence insecticides, the number of treatments could be greatly reduced. The resulting savings in time and money would ena- ble the organization of the treatments of all the palms located in an infested area, and consequently the rapid eradication of the pest. -

A Compilation and Analysis of Food Plants Utilization of Sri Lankan Butterfly Larvae (Papilionoidea)
MAJOR ARTICLE TAPROBANICA, ISSN 1800–427X. August, 2014. Vol. 06, No. 02: pp. 110–131, pls. 12, 13. © Research Center for Climate Change, University of Indonesia, Depok, Indonesia & Taprobanica Private Limited, Homagama, Sri Lanka http://www.sljol.info/index.php/tapro A COMPILATION AND ANALYSIS OF FOOD PLANTS UTILIZATION OF SRI LANKAN BUTTERFLY LARVAE (PAPILIONOIDEA) Section Editors: Jeffrey Miller & James L. Reveal Submitted: 08 Dec. 2013, Accepted: 15 Mar. 2014 H. D. Jayasinghe1,2, S. S. Rajapaksha1, C. de Alwis1 1Butterfly Conservation Society of Sri Lanka, 762/A, Yatihena, Malwana, Sri Lanka 2 E-mail: [email protected] Abstract Larval food plants (LFPs) of Sri Lankan butterflies are poorly documented in the historical literature and there is a great need to identify LFPs in conservation perspectives. Therefore, the current study was designed and carried out during the past decade. A list of LFPs for 207 butterfly species (Super family Papilionoidea) of Sri Lanka is presented based on local studies and includes 785 plant-butterfly combinations and 480 plant species. Many of these combinations are reported for the first time in Sri Lanka. The impact of introducing new plants on the dynamics of abundance and distribution of butterflies, the possibility of butterflies being pests on crops, and observations of LFPs of rare butterfly species, are discussed. This information is crucial for the conservation management of the butterfly fauna in Sri Lanka. Key words: conservation, crops, larval food plants (LFPs), pests, plant-butterfly combination. Introduction Butterflies go through complete metamorphosis 1949). As all herbivorous insects show some and have two stages of food consumtion. -

1 Testing of Garlic Based Bio-Pesticide on Insect Pests of Coconut (Cocus Nucifera L.)
TESTING OF GARLIC BASED BIO-PESTICIDE ON INSECT PESTS OF COCONUT (COCUS NUCIFERA L.) A REPORT 2008-2009 UNIVERSITY OF AGRICULTURAL SCIENCES, GANDHI KRISHI VIGNAN KENDRA BANGALORE-560065 1 TESTING OF GARLIC BASED BIO-PESTICIDE ON INSECT PESTS OF COCONUT (COCUS NUCIFERA L.) A REPORT 2008-2009 UNIVERSITY OF AGRICULTURAL SCIENCES, GANDHI KRISHI VIGNAN KENDRA BANGALORE-560065 2 PRINCIPAL INVESTIGATOR Dr. A. K. CHAKRAVARTHY PROFFESSOR DEPARTMENT OF AGRICULTURAL ENTOMOLOGY UAS, GKVK, BANGALORE-560065 CO-INVESTIGATOR B.DODDABASAPPA COLLEGE OF AGRICULTURE UAS, GKVK, BANGALORE-560065 3 TESTING OF GARLIC BASED BIO-PESTICIDE ON INSECT PESTS OF COCONUT (COCUS NUCIFERA L.) Introduction: In recent days organic farming plays an important role in getting quality food, since people are health conscious and many times asking for organic tender coconut and organic copra. Coconut (Cocus nucifera L.) is one of the important plantation crops cultivated across 19.5 lakh ha in India with a production of 14811 lakh nuts with an average of 7608 nuts. In Southern India, every house uses coconut almost every day for one or the other purpose. In addition to use of nuts for food and in temples for spiritual customs and ceremonies, in many parts of the world coconut oil is used as food, bio-fuel and lubricant. While it is one of the important commercial crops in India, it is the most important crop in the world. Keeping this in background the following objectives were framed to upate the pest management practices through suppressing the pests by bio- pesticides (Muralimohan, et al.,2008). In Karnataka ,this palm which is called Kalpavruksh, accounts for more than 18 percent of area in India, is predominantly grown in three agro-systems- hill and mountain (Districts of Hassan, Tumkur, South Chitradurga, Shimoga and Chikmagalur), Coastal (Mangalore, North Karnataka) and plains (Mysore, Mandya, Bangalore rural, Kolar). -

ISSN 2320-5407 International Journal of Advanced Research (2015), Volume 3, Issue 1, 206-211
ISSN 2320-5407 International Journal of Advanced Research (2015), Volume 3, Issue 1, 206-211 Journal homepage: http://www.journalijar.com INTERNATIONAL JOURNAL OF ADVANCED RESEARCH RESEARCH ARTICLE BUTTERFLY SPECIES DIVERSITY AND ABUNDANCE IN MANIKKUNNUMALA FOREST OF WESTERN GHATS, INDIA. M. K. Nandakumar1, V.V. Sivan1, Jayesh P Joseph1, M. M. Jithin1, M. K. Ratheesh Narayanan2, N. Anilkumar1. 1 Community Agrobiodiversity Centre, M S Swaminathan Research Foundation,Puthoorvayal, Kalpetta, Kerala- 673121, India 2 Department of Botany, Payyanur College, Edat P.O., Kannur, Kerala-670327, India Manuscript Info Abstract Manuscript History: Butterflies, one of the most researched insect groups throughout the world, are also one of the groups that face serious threats of various kinds and in Received: 11 November 2014 Final Accepted: 26 December 2014 varying degrees. Wayanad district is one of the biodiversity rich landscapes Published Online: January 2015 within the biodiversity hot spot of Western Ghats. This paper essentially deals with the abundance and diversity of butterfly species in Key words: Manikkunnumala forest in Wayanad district of Western Ghats. The hilly ecosystem of this area is under various pressures mainly being Butterfly diversity, Abundance, anthropogenic. Still this area exhibits fairly good diversity; this includes Wayanad, Western Ghats some very rare and endemic butterflies. When assessed the rarity and *Corresponding Author abundance, six out of 94 recorded butterflies comes under the Indian Wildlife Protection Act, 1972. The area needs immediate attention to conserve the M. K. Nandakumar remaining vegetation in order to protect the butterfly diversity. Copy Right, IJAR, 2015,. All rights reserved INTRODUCTION Butterflies are one of the unique groups of insects, which grasp the attention of nature lovers worldwide. -

Ecological Studies on Mango Leaf Webber (Orthaga Exvinaceahamp.)
Internat. J. agric. Sci. Vol.2 No.2 July 2006 : (308-311) 308 Ecological studies on mango leaf webber (Orthaga exvinacea Hamp.) in Andhra Pradesh as a basis for IPM M. Kannan*1 and N. Venugopala Rao Department of Entomology, S.V. Agricultural College, TIRUPATI (A.P.) INDIA ABSTRACT The influence of ecological factors viz., biotic (Host plant) and abiotic factors (weather parameters) on the abundance and population fluctuation of leaf webber, Orthaga exvinacea (Hamp.) on mango under the conditions of Chittoor district were worked out. Peak incidence was observed during first fortnight of November (19.4 webs/tree). However, gradual increase was observed from the first fortnight of July (2.6 webs/tree) and declined during second fortnight of January (3.2 webs/tree). Correlation studies between incidence and weather parameters showed positive relationship with minimum temperature, relative humidity and rainfall and negative relationship with maximum temperature. None of the varieties was free from infestation. The infestation ranged from 7.80 to 29.47 webs/tree, 5.82 to 22.55 leaves/web and 1.92 to 29.47 larvae/tree. Variety Neelum showed less infestation, while Bangalore showed severe infestation and other varieties viz., Neeleghan, Cherakurasam, Mulgova, Rumani, Baneshan and Swarnajahangir have moderate infestation. Result of the study also revealed that older mango trees (15 and above years old) were more susceptible (18.26, 348.75, 121.61 webs/tree, webbed leaves/tree and larva/ tree, respectively) to leaf webber damage than young trees (0-5 years old). Key Words: Mango leaf webber, Ecological studies, Varietal susceptibility, Abiotic factors INTRODUCTION Impact of abiotic factors on population dynamics of leaf webber Mango, Mangifera indica is an important fruit crop of India. -

Hemiptera: Miridae: Deraeocorinae) from India with Biological Note
J. Entomol. Res. Soc., 20(3): 67-73, 2018 Research Article Print ISSN:1302-0250 Online ISSN:2651-3579 First Record of Termatophylum orientale Poppius (Hemiptera: Miridae: Deraeocorinae) from India with Biological Note Richa VARSHNEY1* Yeshwanth H. M.2 1 ICAR-National Bureau of Agricultural Insect Resources, P.B. No. 2491, H.A. Farm Post, Bellary Road, Hebbal, Bangalore-560024, INDIA. e-mail: *[email protected] 2 Department of Entomology, University of Agricultural Sciences, GKVK, Bangalore-560065 INDIA. e-mail: [email protected] ABSTRACT Termatophylum orientale Poppius is being reported for the first time from India. It was collected from Mangifera indica (Mango, Anacardiaceae), Carica papaya (Papaya, Caricaceae) and Peltophorum pterocarpum (Copperpod, Fabaceae) where it shares niche along with other predators like anthocorids, geocorids and pests like thrips, mites and lepidopteran larvae. For the first time rearing protocol and biology has been given for this mirid. Key words: Mango, Miridae, Deraeocorinae, Termatophylini, Termatophylum orientale, Thrips. Varshney, R., Yeshwanth, H.M. (2018). First record of Termatophylum orientale Poppius (Hemiptera: Miridae: Deraeocorinae) from India with biological note. Journal of the Entomological Research Society, 20(3), 67-73. 68 VARSHNEY, R., H. M., Y. INTRODUCTION Mirid bugs of the tribe Termatophylini are known to inhabit inflorescences, moth larval galleries or rolled bark. They are known to feed on thrips, besides feeding on nectar and pollen (Cassis, 1995; Cassis et al., 2011; Yasunaga et al., 2001). Three species of the genus Termatophylidea Reuter and Poppius were reported to attack on the cacao thrips, occupying the niche shared by anthocorids. It is assumed that these mirids are obligate predators and feed exclusively on thrips. -
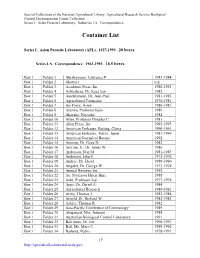
Container List
Special Collections of the National Agricultural Library: Agricultural Research Service Biological Control Documentation Center Collection Series I. Asian Parasite Laboratory. Subseries I.A. Correspondence. Container List Series I. Asian Parasite Laboratory (APL). 1927-1993. 20 boxes. Series I.A. Correspondence. 1963-1993. 16.5 boxes. Box 1 Folder 1 Abrahamson, Lawrence P. 1983-1984 Box 1 Folder 2 Abstract n.d. Box 1 Folder 3 Academic Press, Inc. 1986-1993 Box 1 Folder 4 Achterberg, Dr. Kees van 1982 Box 1 Folder 5 Aeschlimann, Dr. Jean-Paul 1981-1985 Box 1 Folder 6 Agricultural Counselor 1976-1981 Box 1 Folder 7 Air Force, Army 1980-1981 Box 1 Folder 8 Aizawa, Professor Keio 1986 Box 1 Folder 9 Akasaka, Naoyuki 1982 Box 1 Folder 10 Allen, Professor Douglas C. 1981 Box 1 Folder 11 Allen Press, Inc. 1992-1993 Box 1 Folder 12 American Embassy, Beijing, China 1990-1991 Box 1 Folder 13 American Embassy, Tokyo, Japan 1981-1990 Box 1 Folder 14 American Journal of Botany 1992 Box 1 Folder 15 Amman, Dr. Gene D. 1982 Box 1 Folder 16 Amrine, Jr., Dr. James W. 1986 Box 1 Folder 17 Anderson, Don M. 1981-1983 Box 1 Folder 18 Anderson, John F. 1975-1976 Box 1 Folder 19 Andres, Dr. Lloyd 1989-1990 Box 1 Folder 20 Angalet, Dr. George W. 1973-1978 Box 1 Folder 21 Annual Reviews Inc. 1992 Box 1 Folder 22 Ao, Professor Hsien-Bine 1988 Box 1 Folder 23 Aoki, Professor Joji 1977-1978 Box 1 Folder 24 Apps, Dr. Darrel A. 1984 Box 1 Folder 25 Agricultural Research 1980-1981 Box 1 Folder 26 Army, Thomas J. -

Downloaded from BOLD Or Requested from Other Authors
www.nature.com/scientificreports OPEN Towards a global DNA barcode reference library for quarantine identifcations of lepidopteran Received: 28 November 2018 Accepted: 5 April 2019 stemborers, with an emphasis on Published: xx xx xxxx sugarcane pests Timothy R. C. Lee 1, Stacey J. Anderson2, Lucy T. T. Tran-Nguyen3, Nader Sallam4, Bruno P. Le Ru5,6, Desmond Conlong7,8, Kevin Powell 9, Andrew Ward10 & Andrew Mitchell1 Lepidopteran stemborers are among the most damaging agricultural pests worldwide, able to reduce crop yields by up to 40%. Sugarcane is the world’s most prolifc crop, and several stemborer species from the families Noctuidae, Tortricidae, Crambidae and Pyralidae attack sugarcane. Australia is currently free of the most damaging stemborers, but biosecurity eforts are hampered by the difculty in morphologically distinguishing stemborer species. Here we assess the utility of DNA barcoding in identifying stemborer pest species. We review the current state of the COI barcode sequence library for sugarcane stemborers, assembling a dataset of 1297 sequences from 64 species. Sequences were from specimens collected and identifed in this study, downloaded from BOLD or requested from other authors. We performed species delimitation analyses to assess species diversity and the efectiveness of barcoding in this group. Seven species exhibited <0.03 K2P interspecifc diversity, indicating that diagnostic barcoding will work well in most of the studied taxa. We identifed 24 instances of identifcation errors in the online database, which has hampered unambiguous stemborer identifcation using barcodes. Instances of very high within-species diversity indicate that nuclear markers (e.g. 18S, 28S) and additional morphological data (genitalia dissection of all lineages) are needed to confrm species boundaries. -

Journal of the Bombay Natural History Society
' <«» til 111 . JOURNAL OF THE BOMBAY NATURAL HISTORY SOCIETY Hornbill House, Shaheed Bhagat Singh Marg, Mumbai 400 001 Executive Editor Asad R. Rahmani, Ph. D Bombay Natural History Society, Mumbai Copy and Production Editor Vibhuti Dedhia, M. Sc. Editorial Board M.R. Almeida, D. Litt. T.C. Narendran, Ph. D., D. Sc. Bombay Natural History Society, Mumbai Professor, Department of Zoology, University of Calicut, Kerala Ajith Kumar, Ph. D. National Centre for Biological Sciences, GKVK Campus, Aasheesh Pittie, B. Com. Hebbal, Bangalore Bird Watchers Society of Andhra Pradesh, Hyderabad M.K. Chandrashekaran, Ph. D., D. Sc. Nehru Professor, Jawaharlal Centre G.S. Rawat, Ph. D. for Scientific Research, Advanced Wildlife Institute of India, Dehradun Bangalore K. Rema Devi, Ph. D. Anwaruddin Choudhury, Ph. D., D. Sc. Zoological Survey of India, Chennai The Rhino Foundation for Nature, Guwahati J.S. Singh, Ph. D. Indraneil Das, D. Phil. Professor, Banaras Hindu University, Varanasi Institute of Biodiversity and Environmental Conservation, Universiti Malaysia, Sarawak, Malaysia S. Subramanya, Ph. D. University of Agricultural Sciences, GKVK, P.T. Cherian, Ph. D. Hebbal, Bangalore Emeritus Scientist, Department of Zoology, University of Kerala, Trivandrum R. Sukumar, Ph. D. Professor, Centre for Ecological Sciences, Y.V. Jhala, Ph. D. Indian Institute of Science, Bangalore Wildlife Institute of India, Dehrdun K. Ullas Karanth, Ph. D. Romulus Whitaker, B Sc. Wildlife Conservation Society - India Program, Madras Reptile Park and Crocodile Bank Trust, Bangalore, Karnataka Tamil Nadu Senior Consultant Editor J.C. Daniel, M. Sc. Consultant Editors Raghunandan Chundawat, Ph. D. Wildlife Conservation Society, Bangalore Nigel Collar, Ph. D. BirdLife International, UK Rhys Green, Ph.