BRAIN, BEHAVIOR, and IMMUNITY EDITOR-IN-CHIEF Keith W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

PROGRESS in NEUROSCIENCE PINS “CNS Lymphatic Drainage In
PROGRESS IN NEUROSCIENCE PINS Seminar Series of the Brain & Mind Research Institute Weill Cornell Medical College (WCMC) & The Graduate Program in Neuroscience of WCMC and Sloan Kettering Institute Thursday, 12/8/16, 4 PM, coffee at 3:45 PM Uris Auditorium “CNS lymphatic drainage in health and disease” Jonathan Kipnis, Ph.D., Center for Brain Immunology and Glia (BIG), Harrison Distinguished Teaching Professor of Neuroscience and Chair, Department of Neuroscience, University of Virginia Abstract The central nervous system was considered to be devoid of classical lymphatic drainage. We recently challenged that paradigm by demonstrating the presence of a lymphatic vasculature in the surrounding of the brain called the meninges. We demonstrated that lymphatic vessels, expressing the markers for lymphatic endothelial cells (LEC; i.e Lyve-1, Prox1, podoplanin, VEGFR3 and CCL21) are located along the dural sinuses. They present features of initial lymphatics, and, importantly, drain fluids, macromolecules and immune cells from the cerebrospinal fluid and the CNS parenchyma into the deep cervical lymph nodes. Our recent efforts are concentrated on understanding the role of meningeal lymphatic vessels in CNS function in health and disease. Our results suggest that the drainage into the deep cervical lymph nodes might play different roles at different stages of several neurological diseases. Understanding the function of the lymphatic drainage in CNS might shed a new light on neurological disorders and offer new therapeutic targets. Recent Relevant Publications: 1. Gadani SP, Walsh JT, Smirnov I, Zheng J and Kipnis J. (2015) The glia-derived alarmin IL-33 orchestrates the post CNS injury immune response and promotes recovery. -

Quarterly Report: Second Quarter 2017
QUARTERLY REPORT: 2ND QUARTER 2017 Q2 2017 Research Consortium Welcomes Three New Members The Brain’s 2 Phyllis Rappaport Updates Former College Classmates Lymphatic System 3 Modern science has an incredibly thorough understanding of the human body. It is J. McLaughlin’s ‘Sip ’n Shop’ hard to imagine that any organ or system could exist within the body that has yet to be Fundraiser discovered. Yet this is exactly what happened in 2015, when researchers discovered 4 lymphatic vessels around and within the brain. Living with Alzheimer’s Film The lymphatic system exists throughout the body. Part of the immune system, it consists Screening of a network of channels (vessels) and glands called “nodes.” Nodes create immune cells 5 to help the body fight infection. The vessels carry fluid containing these immune cells, as well as pathogens and harmful cellular waste products, away from organs. Until recently, CaringKind Alliance Update lymphatic vessels had never been observed in or around the brain. While there were other 5 known ways—such as immune cells called macrophages—for waste products to be Extra Sets of Hands cleared from the brain, researchers had trouble explaining the volume of clearance they observed without a lymphatic system. 5 Cure Alzheimer’s Fund A recent study at the University of Virginia by Jonathan Kipnis, Ph.D., and his colleagues Heroes Antoine Louveau, Ph.D., and Tajie Harris, Ph.D., shed new light on this problem. Using a new method of examining the meninges, a membrane that covers the brain, they 6 discovered there was in fact a network of lymphatic vessels surrounding the brain. -

Massachusetts Licensed Motor Vehicle Damage Appraisers - Individuals September 05, 2021
COMMONWEALTH OF MASSACHUSETTS DIVISION OF INSURANCE PRODUCER LICENSING 1000 Washington Street, Suite 810 Boston, MA 02118-6200 FAX (617) 753-6883 http://www.mass.gov/doi Massachusetts Licensed Motor Vehicle Damage Appraisers - Individuals September 05, 2021 License # Licensure Individual Address City State Zip Phone # 1 007408 01/01/1977 Abate, Andrew Suffolk AutoBody, Inc., 25 Merchants Dr #3 Walpole MA 02081 0-- 0 2 014260 11/24/2003 Abdelaziz, Ilaj 20 Vine Street Lexington MA 02420 0-- 0 3 013836 10/31/2001 Abkarian, Khatchik H. Accurate Collision, 36 Mystic Street Everett MA 02149 0-- 0 4 016443 04/11/2017 Abouelfadl, Mohamed N Progressive Insurance, 2200 Hartford Ave Johnston RI 02919 0-- 0 5 016337 08/17/2016 Accolla, Kevin 109 Sagamore Ave Chelsea MA 02150 0-- 0 6 010790 10/06/1987 Acloque, Evans P Liberty Mutual Ins Co, 50 Derby St Hingham MA 02018 0-- 0 7 017053 06/01/2021 Acres, Jessica A 0-- 0 8 009557 03/01/1982 Adam, Robert W 0-- 0 West 9 005074 03/01/1973 Adamczyk, Stanley J Western Mass Collision, 62 Baldwin Street Box 401 MA 01089 0-- 0 Springfield 10 013824 07/31/2001 Adams, Arleen 0-- 0 11 014080 11/26/2002 Adams, Derek R Junior's Auto Body, 11 Goodhue Street Salem MA 01970 0-- 0 12 016992 12/28/2020 Adams, Evan C Esurance, 31 Peach Farm Road East Hampton CT 06424 0-- 0 13 006575 03/01/1975 Adams, Gary P c/o Adams Auto, 516 Boston Turnpike Shrewsbury MA 01545 0-- 0 14 013105 05/27/1997 Adams, Jeffrey R Rodman Ford Coll Ctr, Route 1 Foxboro MA 00000 0-- 0 15 016531 11/21/2017 Adams, Philip Plymouth Rock Assurance, 901 Franklin Ave Garden City NY 11530 0-- 0 16 015746 04/25/2013 Adams, Robert Andrew Country Collision, 20 Myricks St Berkley MA 02779 0-- 0 17 013823 07/31/2001 Adams, Rymer E 0-- 0 18 013999 07/30/2002 Addesa, Carmen E Arbella Insurance, 1100 Crown Colony Drive Quincy MA 02169 0-- 0 19 014971 03/04/2008 Addis, Andrew R Progressive Insurance, 300 Unicorn Park Drive 4th Flr Woburn MA 01801 0-- 0 20 013761 05/10/2001 Adie, Scott L. -
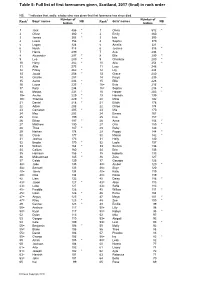
Table 5: Full List of First Forenames Given, Scotland, 2016 (Final) in Rank Order
Table 5: Full list of first forenames given, Scotland, 2017 (final) in rank order NB: * indicates that, sadly, a baby who was given that first forename has since died. Number of Number of Rank1 Boys' names NB Rank1 Girls' names NB babies babies 1 Jack 486 * 1 Olivia 512 * 2 Oliver 380 * 2 Emily 460 3 James 368 * 3 Isla 395 4 Lewis 356 4 Sophie 370 5 Logan 324 5 Amelia 321 6 Noah 318 6 Jessica 318 * 7 Harris 299 7 Ava 294 8 Alexander 297 * 8 Ella 290 * 9 Leo 289 * 9 Charlotte 280 * 10 Harry 282 * 10 Aria 254 * 11 Alfie 275 11 Lucy 248 12 Finlay 262 * 12 Lily 244 13 Jacob 258 * 13 Grace 240 14 Charlie 257 14 Freya 235 15 Aaron 236 * 15 Ellie 228 16 Lucas 235 * 16= Evie 216 17 Rory 234 16= Sophia 216 * 18 Mason 231 * 18 Harper 203 * 19= Archie 229 * 19 Hannah 199 19= Thomas 229 * 20 Millie 192 21 Daniel 218 * 21 Eilidh 178 22 Adam 208 22 Chloe 174 23 Cameron 205 * 23 Mia 170 24 Max 203 24 Emma 167 25 Finn 199 25 Eva 157 26 Ethan 197 * 26 Anna 154 * 27 Matthew 190 27 Orla 150 * 28 Theo 187 * 28 Ruby 146 29 Nathan 178 29 Poppy 144 * 30 Oscar 177 30 Maisie 142 * 31 Joshua 173 31 Holly 140 32 Brodie 170 * 32 Layla 137 33 William 164 * 33 Sienna 136 34 Callum 160 34 Erin 135 35 Harrison 156 * 35 Isabella 133 36 Muhammad 145 * 36 Zara 127 37 Caleb 139 37 Georgia 126 38= Jude 135 38= Amber 120 * 38= Samuel 135 38= Skye 120 40= Jamie 134 40= Katie 119 40= Ollie 134 40= Rosie 119 42 Liam 132 42 Daisy 116 * 43= Jaxon 127 * 43= Alice 113 43= Luke 127 43= Sofia 113 45= Freddie 126 45 Willow 111 * 45= Isaac 126 * 46 Esme 104 47= Angus 125 47 Maya 101 -

A Strategic Investment in Pediatrics and Infectious Diseases Dr
December 2020 milestones Dr. Sallie Permar, the new chair of Weill Cornell Medicine’s Department of Pediatrics A Strategic Investment in Pediatrics and Infectious Diseases Dr. Sallie Permar, a distinguished physician-scientist who of such viruses as HIV, Zika and cytomegalovirus (CMV), the specializes in pediatric infectious diseases, joined Weill Cornell most common congenital infection and a leading cause of birth Medicine on December 1 as the new chair of the Department defects. In her research, she also discovered a protein in breast of Pediatrics. milk that neutralizes HIV, the virus that causes AIDS. The recruitment of Dr. Permar is part of Weill Cornell Medicine’s “Dr. Permar will enhance our mission in both pediatrics and strategic investment in pediatrics and infectious disease research infectious diseases, building on our wealth of research as she and clinical care, with a goal of raising nearly $60 million to support collaborates with investigators and clinicians to improve the expanded translational research efforts in the Belfer Research lives of children,” says Dr. Augustine M.K. Choi, the Stephen and Building. The COVID-19 pandemic has reinforced the growing need Suzanne Weiss Dean. “As a leading academic medical center, for research of infectious diseases of all types – including areas in we must expand our investment in infectious diseases with an which Dr. Permar specializes. She and her team are working on the eye toward future global pathogens that can have a profound development of vaccines to prevent mother-to-child transmission impact on human health.” continued on page 2 A Strategic Investment in Pediatrics and Infectious Diseases continued from cover Infectious disease experts at Well Cornell Medicine more than $3 million to establish the Gerald M. -

IN the UNITED STATES COURT of FEDERAL CLAIMS OFFICE of SPECIAL MASTERS No. 01-162V Filed: February 12, 2009 to Be Published
IN THE UNITED STATES COURT OF FEDERAL CLAIMS OFFICE OF SPECIAL MASTERS No. 01-162V Filed: February 12, 2009 To Be Published * * * * * * * * * * * * * * * * * * * * * * * * * * * * COLTEN SNYDER, by and through * KATHRYN SNYDER and JOSEPH SNYDER, * his natural guardians and next friends * Omnibus Autism * Proceeding; * Autism Spectrum Disorder, * Pervasive Developmental * Disorder, Causation, Petitioners, * Measles, MMR, Mercury, * Thimerosal, Waiver v. * Applying Daubert, * Weight of Expert Opinions, SECRETARY OF THE DEPARTMENT * Credibility of Witnesses OF HEALTH AND HUMAN SERVICES, * * Respondent. * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * Christopher W. Wickersham, Sr., Esq., Lloyd Bowers, Esq., and Thomas B. Powers, Esq., for petitioners. Alexis S. Babcock, Esq., Katherine Esposito, Esq., Voris Johnson, Esq., and Vincent Matanoski, Esq., U.S. Department of Justice, Washington, DC, for respondent. DECISION1 Vowell, Special Master: On March 22, 2001, Kathryn and Joseph Snyder [“petitioners”] filed a petition for compensation under the National Vaccine Injury Compensation Program, 42 U.S.C. 1 Vaccine Rule 18(b) provides the parties 14 days to request redaction of any material “(i) which is trade secret or commercial or financial information which is privileged and confidential, or (ii) which are medical files and similar files, the disclosure of which would constitute a clearly unwarranted invasion of privacy.” 42 U.S.C. § 300aa12(d)(4)(B). Petitioners have waived their right to request such redaction. See Petitioners’ Notice to W aive the 14-Day “W aiting” Period as Defined in Vaccine Rule 18(b), filed December 2, 2008. Respondent also waived the right to object to the disclosure of information submitted by respondent. See Respondent’s Consent to Disclosure, filed January 14, 2009. Accordingly, this decision will be publicly available immediately after it is filed. -

2018 Winter Newsletter
Holiday Gift Ideas A Q&A wth Alan Arnette Plan Your Giving Now Celebrate the reason for the season Arnette is a mountaineer, speaker Make giving a commitment with a gift that gives back and Alzheimer’s advocate to Cure Alzheimer's Fund WINTER SEASON 2018 THE CASE FOR HOPE Panel Explores the Path Forward in Alzheimer’s Research at Our 8th Annual Scientific Symposium THE CASE FOR HOPE SCIENCE PANEL EXPLORES THE PATH FORWARD IN ALZHEIMER’S RESEARCH The 8th Annual Cure Alzheimer’s Fund Symposium featured award-winning NPR science writer Jon Hamilton moderating a discussion between Drs. Ron Petersen, Bob Vassar and Teresa Gomez-Isla on the Case For Hope in Alzheimer’s disease research. Co-Chairman Jeff Morby started the meeting by announcing that for the frst time. She remembered being impacted by learning Cure Alzheimer’s Fund had just received the designation of Top that the disease can steal one of the “greatest treasures we have 10 Best Medical Research Organizations by nonproft watchdog as human beings—our memories.” Charity Navigator. After providing the audience with an update of CureAlz's results, including $75 million distributed for 340 Progressing Through Challenges research grants to 127 of the world’s leading researchers, he then introduced Dr. Rudy Tanzi, who introduced the panel: Dr. Petersen The panelists described the challenge of connecting the events as a champion for scientists whose policy work has been pav- happening in the brain to the symptoms experienced by patients. ing the way for clinical trials that target amyloid before the onset All three of the scientists explained that amyloid plaques and of symptoms, Dr. -

3040 Clairemont Drive Apartments
DATE ISSUED: September 20, 2018 REPORT NO. PC-18-060 HEARING DATE: September 27, 2018 SUBJECT: 3040 CLAIREMONT DRIVE APARTMENTS. Process Four Decision PROJECT NUMBER: 410740 OWNER/APPLICANT: Sam and Sandra Dimenstein Owners/Scott Spencer and Associates Applicant SUMMARY Issue: Should the Planning Commission approve the construction of a 35,240-square-foot, three-story, 19-unit apartment building and a 1,500-square-foot commercial building located at 3040 Clairemont Drive in the Clairemont Mesa Community Planning area. Staff Recommendations: 1. Approve Site Development Permit No. 1835923, and 2. Approve Planned Development Permit No. 143717, and 3. Approve Easement Vacation No. 2189558. Community Planning Group Recommendation: On October 17, 2017 the Clairemont Community Planning Group voted 9-4-0 to recommend approval of the project with no conditions. Environmental Review: The project was determined to be categorically exempt from the California Environmental Quality Act (CEQA) pursuant to Section 15332 (In-Fill Development Projects). This project is not pending an appeal of the environmental determination. The environmental exemption determination for this project was made on April 19, 2018, and the opportunity to appeal that determination ended on May 3, 2018. Fiscal Impact Statement: None with this action. All costs associated with the processing of the project are paid from a deposit account maintained by the applicant. Code Enforcement Impact: None. Housing Impact Statement: The Clairemont Mesa Community Plan designates the 3.318- acre site for mixed use development. The project would comply with that designation and is proposing 19 apartment units within the three-story residential building and a one-story commercial building. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub
US 20090005722A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub. Date: Jan. 1, 2009 (54) SKIN-CONTACTING-ADHESIVE FREE Publication Classification DRESSING (51) Int. Cl. Inventor: Barbara Jennings-Spring, Jupiter, A61N L/30 (2006.01) (76) A6F I3/00 (2006.01) FL (US) A6IL I5/00 (2006.01) Correspondence Address: AOIG 7/06 (2006.01) Irving M. Fishman AOIG 7/04 (2006.01) c/o Cohen, Tauber, Spievack and Wagner (52) U.S. Cl. .................. 604/20: 602/43: 602/48; 4771.5; Suite 2400, 420 Lexington Avenue 47/13 New York, NY 10170 (US) (57) ABSTRACT (21) Appl. No.: 12/231,104 A dressing having a flexible sleeve shaped to accommodate a Substantially cylindrical body portion, the sleeve having a (22) Filed: Aug. 29, 2008 lining which is substantially non-adherent to the body part being bandaged and having a peripheral securement means Related U.S. Application Data which attaches two peripheral portions to each other without (63) Continuation-in-part of application No. 1 1/434,689, those portions being circumferentially adhered to the sleeve filed on May 16, 2006. portion. Patent Application Publication Jan. 1, 2009 Sheet 1 of 9 US 2009/0005722 A1 Patent Application Publication Jan. 1, 2009 Sheet 2 of 9 US 2009/0005722 A1 10 8 F.G. 5 Patent Application Publication Jan. 1, 2009 Sheet 3 of 9 US 2009/0005722 A1 13 FIG.6 2 - Y TIII Till "T fift 11 10 FIG.7 8 13 6 - 12 - Timir" "in "in "MINIII. -

Immune Dysfunction and Autoimmunity As Pathological Mechanisms in Autism Spectrum Disorders
REVIEW published: 13 November 2018 doi: 10.3389/fncel.2018.00405 Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders Heather K. Hughes 1,2, Emily Mills Ko 1,2, Destanie Rose 1,2 and Paul Ashwood 1,2* 1 Department of Medical Microbiology and Immunology, University of California, Davis, Davis, CA, United States, 2 MIND Institute, UC Davis Medical Center, Sacramento, CA, United States Autism spectrum disorders (ASD) are a group of heterogeneous neurological disorders that are highly variable and are clinically characterized by deficits in social interactions, communication, and stereotypical behaviors. Prevalence has risen from 1 in 10,000 in 1972 to 1 in 59 children in the United States in 2014. This rise in prevalence could be due in part to better diagnoses and awareness, however, these together cannot solely account for such a significant rise. While causative connections have not been proven in the majority of cases, many current studies focus on the combined effects of genetics and environment. Strikingly, a distinct picture of immune dysfunction has emerged and been supported by many independent studies over the past decade. Many players in the immune-ASD puzzle may be mechanistically contributing to pathogenesis of these disorders, including skewed cytokine responses, differences in total numbers and frequencies of immune cells and their subsets, neuroinflammation, and adaptive and Edited by: innate immune dysfunction, as well as altered levels of immunoglobulin and the presence Jijun Li, Shanghai Jiao Tong University, China of autoantibodies which have been found in a substantial number of individuals with Reviewed by: ASD. This review summarizes the latest research linking ASD, autoimmunity and immune Andreas Martin Grabrucker, dysfunction, and discusses evidence of a potential autoimmune component of ASD. -

Different Minds
DIFFERENT MINDS if there’s one thing Ralph Or not. the fact that there are few AN INFECTIOUS THEORY Adolphs wants you to under- absolutes is part of why the set of dis- Much of that insight comes from Pat- stand about autism, it’s this: “it’s orders—autism, Asperger’s, pervasive terson, who pioneered the study of the wrong to call many of the people developmental disorder not otherwise connections between the brain and the on the autism spectrum impaired,” says specified (PDD-NOS)—that fall under immune system in autism, schizophrenia, the Caltech neuroscientist. “they’re autism’s umbrella are referred to as and depression a decade ago. simply different.” a spectrum. As with the spectrum of the main focus of that connection? these differences are in no way insig- visible light—where red morphs into Some kind of viral infection during preg- nificant—they are, after all, why so much orange, which morphs into yellow— nancy, Patterson explains. Or, rather, the effort and passion is being put into un- it is difficult to draw sharp lines immune response that infection inevitably derstanding autism’s most troublesome between the various diagnoses in engenders. traits—but neither are they as inevitably the autism spectrum. to bolster his argument, Patterson devastating as has often been depicted. And, like the autism spectrum itself, points to a recent study by hjordis O. they are simply differences; intriguing, the spectrum of autism research at Atladottir of Aarhus University, Denmark, fleeting glimpses into minds that work in Caltech also runs a gamut. Adolphs, and colleagues—“an extraordinary look at ways most of us don’t quite understand, for example, studies brain differences over 10,000 autism cases” in the Danish and yet which may ultimately give each between adults on the high-functioning Medical Register, which is a comprehen- and every one of us a little more insight portion of the autism spectrum and the sive database of every Dane’s medical into our own minds, our own selves.