Odontogenic Epithelial Stem Cells: Hidden Sources
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deciduous Teeth Overview
Deciduous Teeth Overview: ● Deciduous teeth are the first set of teeth a person has. They are a total of 20. ● It is important to learn about the teething stage to help make it a less painful and irritating experience for children. ● Most common problems for deciduous teeth: Caries (cavities), pain and infection, thumb sucking and using a pacifier for longer than the average age. ● A child’s face and teeth may also be injured, affecting the permanent tooth that would replace the injured primary tooth. ● Care guidelines for children’s teeth and mouths should be followed and taken seriously. What are primary teeth? They are the first set of teeth a person has and they remain until it is time for them to fall and be replaced by permanent teeth. Number: 20 teeth Other names: Baby teeth, shed teeth, temporary teeth, primary teeth, milk teeth. Importance of deciduous teeth: ● They help the child chew food. ● They aid speed and enunciation. ● Primary teeth occupy a place in the mouth so they can later allow permanent teeth to appear in their correct place. When a child loses a primary tooth prematurely, this may affect the shape and order of the permanent teeth. ● They help with a child’s aesthetic and increase confidence while smiling When do deciduous teeth appear and when do they shed? Deciduous teeth start appearing gradually starting the age of 6-7 months, beginning with the lower jaw. They are fully developed at the age of 2.5. Development of deciduous teeth (teething): Teething is when a child starts to develop his/her first teeth. -

Journal of Dental Research
Journal of Dental Research http://jdr.sagepub.com/ Cell Differentiation and Matrix Organization in Engineered Teeth A. Nait Lechguer, M.L. Couble, N. Labert, S. Kuchler-Bopp, L. Keller, H. Magloire, F. Bleicher and H. Lesot J DENT RES 2011 90: 583 originally published online 4 February 2011 DOI: 10.1177/0022034510391796 The online version of this article can be found at: http://jdr.sagepub.com/content/90/5/583 Published by: http://www.sagepublications.com On behalf of: International and American Associations for Dental Research Additional services and information for Journal of Dental Research can be found at: Email Alerts: http://jdr.sagepub.com/cgi/alerts Subscriptions: http://jdr.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Apr 13, 2011 OnlineFirst Version of Record - Feb 4, 2011 What is This? Downloaded from jdr.sagepub.com at Service Commun de la Documentation Université de Strasbourg on September 6, 2013 For personal use only. No other uses without permission. © 2011 International & American Associations for Dental Research RESEARCH REPORTS Biomaterials & Bioengineering A. Nait Lechguer1,2, M.L. Couble3,4, N. Labert3,4, S. Kuchler-Bopp1,2, Cell Differentiation and L. Keller1,2, H. Magloire3,4, F. Bleicher3,4, Matrix Organization in and H. Lesot1,2* Engineered Teeth 1INSERM UMR 977, Faculté de Médecine, 11, rue Humann, F-67085 Strasbourg, France; 2Dental School, University of Strasbourg, Strasbourg, France; 3Université de Lyon, Faculté d’Odontologie, Rue Guillaume Paradin, F-69372 Lyon Cedex 08, France; and 4IGFL, CNRS UMR 5242, Ecole Normale Supérieure, 46 Allée d’Italie, 69364, Lyon Cedex 08, France; *corresponding author, [email protected] J Dent Res 90(5):583-589, 2011 ABSTRACT InTRODuCTIOn Embryonic dental cells were used to check a series of criteria to be achieved for tooth engineering. -

6 Development of the Teeth: Root and Supporting Structures Nagat M
AVERY Chap.06 27-11-2002 10:09 Pagina 108 108 II Development of the Teeth and Supporting Structures 6 Development of the Teeth: Root and Supporting Structures Nagat M. ElNesr and James K. Avery Chapter Outline Introduction Introduction... 108 Objectives... 108 Root development is initiated through the contributions Root Sheath Development... 109 of the cells originating from the enamel organ, dental Single-Root Formation... 110 papilla, and dental follicle. The cells of the outer enamel Multiple-Root Formation... 111 epithelium contact the inner enamel epithelium at the Root Formation Anomalies... 112 base of the enamel organ, the cervical loop (Figs. 6.1 and Fate of the Epithelial Root Sheath (Hertwig's Sheath)... 113 6.2A). Later, with crown completion, the cells of the cer- Dental Follicle... 114 vical loop continue to grow away from the crown and Development of (Intermediate) Cementum... 116 become root sheath cells (Figs. 6.2B and 6.3). The inner Cellular and Acellular Cementum... 116 root sheath cells cause root formation by inducing the Development of the Periodontal Ligament... 117 adjacent cells of the dental papilla to become odonto- Development of the Alveolar Process... 119 blasts, which in turn will form root dentin. The root Summary... 121 sheath will further dictate whether the tooth will have Self-Evaluation Review... 122 single or multiple roots. The remainder of the cells of the dental papilla will then become the cells of the root pulp.The third compo- nent in root formation, the dental follicle, is the tissue that surrounds the enamel organ, the dental papilla, and the root. -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Specialized Stem Cell Niche Enables Repetitive Renewal of Alligator Teeth
Specialized stem cell niche enables repetitive renewal PNAS PLUS of alligator teeth Ping Wua, Xiaoshan Wua,b, Ting-Xin Jianga, Ruth M. Elseyc, Bradley L. Templed, Stephen J. Diverse, Travis C. Glennd, Kuo Yuanf, Min-Huey Cheng,h, Randall B. Widelitza, and Cheng-Ming Chuonga,h,i,1 aDepartment of Pathology, University of Southern California, Los Angeles, CA 90033; bDepartment of Oral and Maxillofacial Surgery, Xiangya Hospital, Central South University, Hunan 410008, China; cLouisiana Department of Wildlife and Fisheries, Rockefeller Wildlife Refuge, Grand Chenier, LA 70643; dEnvironmental Health Science and eDepartment of Small Animal Medicine and Surgery, University of Georgia, Athens, GA 30602; fDepartment of Dentistry and iResearch Center for Wound Repair and Regeneration, National Cheng Kung University, Tainan City 70101, Taiwan; and gSchool of Dentistry and hResearch Center for Developmental Biology and Regenerative Medicine, National Taiwan University, Taipei 10617, Taiwan Edited by Edward M. De Robertis, Howard Hughes Medical Institute/University of California, Los Angeles, CA, and accepted by the Editorial Board March 28, 2013 (received for review July 31, 2012) Reptiles and fish have robust regenerative powers for tooth renewal. replaced from the dental lamina connected to the lingual side of However, extant mammals can either renew their teeth one time the deciduous tooth (15). Human teeth are only replaced one time; (diphyodont dentition) or not at all (monophyodont dentition). however, a remnant of the dental lamina still exists (16) and may Humans replace their milk teeth with permanent teeth and then become activated later in life to form odontogenic tumors (17). lose their ability for tooth renewal. -
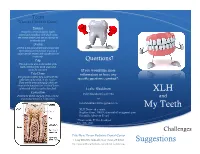
Teeth What Do I Need to Know?
Teeth What do I Need to Know? Enamel Enamel is a semitranslucent, highly mineralized crystalline solid which covers the crowns of teeth and acts as a barrier to protect the teeth. Dentin Dentin is less mineralized than enamel, but more mineralized than bone; it acts as a cushion for the enamel and a further barrier to the pulp. Pulp Questions? The pulp is the area in the middle of the tooth containing the blood vessels and nerves for that tooth. If you would like more Pulp Horns information or have any The projections of the pulp underneath the taller parts of the tooth, or the “cusps.” specific questions, contact*: These are the areas of the pulp which are closest to the functional (or “occlusal”) part of the tooth which is used to chew food. Leslie Blackburn Cementum XLH [email protected] Protects the dentin and pulp of the roots the and way enamel protects it in the crown. or [email protected] My Teeth XLH Network contact: Raghbir Kaur, DMD; [email protected] Scientific Advisory Board *Please include XLH in the subject line of the email. Challenges Yale-New Haven Pediatric Dental Center 1 Long Wharf Dr, Suite 403, New Haven, CT 06510 Suggestions http://www.ynhh.org/medical-services/dental_pediatric.aspx What is the Most Important Thing to Know? It is not your fault. People with XLH have unique dental challenges. Sometimes even when you are doing everything right you may still have dental problems. While it is important to do everything you can to keep your mouth healthy, it is also important to remember that you some things about your oral health are out of your control. -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Gene Expression Profiles in Dental Follicles from Patients with Impacted
Odontology https://doi.org/10.1007/s10266-018-0342-9 ORIGINAL ARTICLE Gene expression profles in dental follicles from patients with impacted canines Pamela Uribe1 · Lena Larsson2 · Anna Westerlund1 · Maria Ransjö1 Received: 9 August 2017 / Accepted: 27 December 2017 © The Author(s) 2018. This article is an open access publication Abstract Animal studies suggest that the dental follicle (DF) plays a major role in tooth eruption. However, the role of the DF during tooth impaction and related root resorptions in adjacent teeth is not clear. The hypothesis for the present study is that expres- sion of regulatory factors involved in the bone remodelling process necessary for tooth eruption may difer between dental follicles from teeth with diferent clinical situations. We have analysed the gene expression profles in the DF obtained from impacted canines, with (N = 3) or without (N = 5) signs of root resorption, and from control teeth (normal erupting teeth, mesiodens) (N = 3). DF from 11 patients (mean age: 13 years) obtains at the time of surgical exposure of the tooth. Due to the surgical time point, all teeth were in a late developmental stage. Gene expression related to osteoblast activation/bone formation, osteoclast recruitment and activation was analysed by RTqPCR. Genes related to bone formation (RUNX2, OSX, ALP, OCN, CX43) were highly expressed in all the samples, but osteoclast recruitment/activation markers (OPG, RANKL, MCP-1, CSF-1) were negligible. No apparent patterns or signifcant diferences in gene expression were found between impacted canines, with or without signs of root resorption, or when compared to control teeth. Our results suggest the DF regulation of osteoclastic activity is limited in the late pre-emergent stage of tooth development, irrespective if the tooth is normally erupting or impacted. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

Pulpotomy Treatment for Primary Teeth
2010 National Primary Oral Health Conference October 24-27 Gaylord Palm, Orlando, Florida Pulpotomy treatment for primary teeth Enrique Bimstein Professor of Pediatric Dentistry University of Florida College of Dentistry. Pulpotomy treatment for primary teeth Goal The participants will become familiar with the basic knowledge and procedures required for the performance of the pulpotomy treatment in primary teeth. Pulpotomy treatment for primary teeth Topics Introduction Definition and rationale. Indications and contraindications. Materials and techniques. Pulpotomy technique (clinical procedures). Pulpotomy follow up. Summary and conclusions. Pulpotomy treatment for primary teeth Topics Introduction Definition and rationale. Indications and contraindications. Materials and techniques. Pulpotomy technique (clinical procedures). Pulpotomy follow up. Summary and conclusions. Preservation of the primary teeth until their time of exfoliation is required to: a. Maintain arch length, masticatory function and esthetics. Preservation of the primary teeth until their time of exfoliation is required to: a. Maintain arch length, masticatory function and esthetics. Preservation of the primary teeth until their time of exfoliation is required to: a. Maintain arch length, masticatory function and esthetics. b. Eliminate pain, inflammation and infection. Preservation of the primary teeth until their time of exfoliation is required to: a. Maintain arch length, masticatory function and esthetics. b. Eliminate pain, inflammation and infection. c. Prevent any additional pain or damage to the oral tissues. Despite all the prevention strategies, childhood caries is still a fact that we confront every day in the clinic. The retention of pulpally involved primary teeth until the time of normal exfoliation remains to be a challenge. Primary teeth with cariously exposed vital pulps should be treated with pulp therapies that allow for the normal exfoliation process. -

Periodontal and Dental Follicle Collagen in Tooth Eruption
SCIENTIFIC ARCHIVES OF DENTAL SCIENCES (ISSN: 2642-1623) Volume 4 Issue 1 January 2021 Review Article Periodontal and Dental Follicle Collagen in Tooth Eruption Norman Randall Thomas* Professor Emeritus, Faculty of Medicine and Dentistry, University of Alberta, Canada *Corresponding Author: Norman Randall Thomas, Professor Emeritus, Faculty of Medicine and Dentistry, University of Alberta, Canada. Received: September 18, 2020; Published: October 20, 2020 Abstract occlusal position in the oral cavity while passive eruption occurs by loss of epithelial attachment to expose the clinical crown. Rodent Review of the process and mechanism of tooth eruption defines active eruption as coronal migration of the tooth to the functional teeth are considered excellent analogs of eruption because they have examples of limited and continuous eruption in the molar teeth and incisors respectively. Root resection studies on rat incisors exhibit normal active eruption rates due to a ‘force’ in the retained prime mover of eruption. Impeded and unimpeded eruption rates were grossly retarded when a collagen crosslinking inhibitor periodontal ligament (PDL). Since all four walls of the tooth and bone remain patent it confirms that the periodontium alone is the lathyrogen 0.3% AAN (aminoacetonitrile) was added to the drinking water of young 45 - 50 gm rats. Using the Bryer 1957 method of measurement of eruption it appeared that low concentrations (0.01%) lathyrogen in the drinking water of adult rats did not intrusion and dilaceration of the reference molar and incisor decreases impeded eruption in the lathyritic condition giving a false have significant retardation of unimpeded eruption rates. Histological, radiological, bone and tooth marker studies indicate that impression of increased unimpeded eruption. -

Cell Delamination in the Mesencephalic Neural Fold and Its
© 2013. Published by The Company of Biologists Ltd | Development (2013) 140, 4890-4902 doi:10.1242/dev.094680 RESEARCH ARTICLE Cell delamination in the mesencephalic neural fold and its implication for the origin of ectomesenchyme Raymond Teck Ho Lee1, Hiroki Nagai2, Yukiko Nakaya2, Guojun Sheng2, Paul A. Trainor3,4, James A. Weston5 and Jean Paul Thiery1,6,7,* ABSTRACT dorsal neural fold epithelia (Hörstadius, 1950) suggested that the The neural crest is a transient structure unique to vertebrate embryos neural crest is the source of multipotent stem cells that give rise to that gives rise to multiple lineages along the rostrocaudal axis. In a remarkable diversity of cell types, including pigment cells, neurons cranial regions, neural crest cells are thought to differentiate into and glia of the peripheral and enteric nervous systems. In addition, chondrocytes, osteocytes, pericytes and stromal cells, which are the neural crest was widely considered to be the source of collectively termed ectomesenchyme derivatives, as well as pigment mesenchymal connective tissues that entered the branchial arches to and neuronal derivatives. There is still no consensus as to whether form components of the craniofacial skeleton and connective tissue the neural crest can be classified as a homogenous multipotent of the dorsal fin at the trunk axial levels of fishes and amphibia population of cells. This unresolved controversy has important (Hörstadius, 1950; Le Douarin and Kalcheim, 1999; Raven, 1936). implications for the formation of ectomesenchyme and for Grafting studies in avian embryos (Le Douarin and Teillet, 1974; confirmation of whether the neural fold is compartmentalized into Nakamura and Ayer-le Lievre, 1982) suggested that developmental distinct domains, each with a different repertoire of derivatives.