1 – Pathogenesis of Pulp and Periapical Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surgical Approaches of Extensive Periapical Cyst
SURGICAL APPROACHES OF EXTENSIVE PERIAPICAL CYST. CONSIDERATIONS ABOUT SURGICAL TECHNIQUE Paulo Domingos Ribeiro Jr.1 Eduardo Sanches Gonçalves1 Eduardo Simioli Neto2 Murilo Rizental Pacenko3 1MSc in R I B E I RO, Paulo Domingos Jr. et al. Surgical approaches of ex t e n s ive Buccomaxilofacial p e r i a p i c a l cyst. Considerations about surgical technique. S a l u s v i t a , surgery and trauma - B a u r u, v. 23, n. 2, p. 317-328, 2004. tology. Dept. of Biological Sciences and Health ABSTRACT Professions – University of the Cystic lesions are frequent in the oral cavity. They are defined as a Sacred Heart, Bauru pathologic cavity with or without fluid or semi fluid material. The – SP. inflammatory lesions are more common, such as periapical cysts. These lesions are encountered in dental apex and the pulp necro s i s 2Graduation course on is a very important cause of these cysts. The treatment can be Buccomaxilofacial c o n s e r v a t i v e, like a biomechanic preparation of root, used when the surgery and lesion is localized, or the surgical treatment, like total or partial traumatology lesion re m oval. When the surgical treatment is realized, the – University of the Sacred Heart, m a r s u p i a l i z a t i o n or decompression can be done before, and an Bauru – SP. enucleation after if necessary, and can be done a total enucleation that enucleate the lesion in one surge r y. -

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -
Small Dog, Big Smile
Small Dog, Big Smile How to make sure your little dog has a happy, healthy mouth Christine Hawke Sydney Pet Dentistry INTRODUCTION Hello and thanks for downloading my book, ‘Small Dog, Big Smile – How to make sure your little dog has a happy, healthy mouth’. Small dogs are gorgeous, and deservedly very popular throughout the world. Centuries of careful and selective breeding has provided us with a huge range of small breeds to choose from, each with their own specific characteristics and charm. While good things certainly come in small packages, one of the drawbacks of their small size is that many of these dogs suffer silently from serious dental issues. While all dog breeds are susceptible to ‘teething problems’, periodontal infection and orthodontic disorders, dogs with small mouths have the added issue of overcrowded teeth to contend with. Similar to their larger ancestors, they have to find space for 42 teeth, which is sometimes no easy feat. As a result, things don’t always go according to plan…. My name is Christine Hawke, and I am a veterinarian with almost 20 years experience in small animal practice. After many years in general practice, I developed a passion for all things dental, and have been running a small animal dentistry-only practice in Sydney since 2007. I am a Member of the Australian and New Zealand College of Veterinary Scientists in the field of Veterinary Dentistry (this can only be attained through examination), and the American Veterinary Dental Society. I am currently the President of the Australian Veterinary Dental Society, and teach veterinary dentistry to vet students (at The University of Sydney), vets and nurses across Australia. -

Clinical SHOWCASE Unintentional Replantation: a Technique to Avoid
Clinical SHOWCASE Unintentional Replantation: A Technique to Avoid Robert S. Roda, DDS, MS any times in a dentist’s career, he the greatest contour of the alveolar or she will make a decision that swelling was over the upper left cuspid. Mhas unintended consequences. In Both teeth had been prepared as bridge the case reported here, some quick abutments, but the temporary bridge was thinking was required to resolve the out- not present. There was an open come of an unexpected series of events. endodontic access in the premolar with Because clinical learning is best achieved no pulp exposure and a small composite by retrospective analysis, a list of lessons resin restoration in the cuspid. Both the to be learned from this case is also pro- cuspid and the second premolar were vided, in the hope that it helps readers to tender to percussion. The cuspid was also avoid this particular situation. very tender to bite (determined with a Tooth Slooth instrument, Professional Case Report Results Inc, Laguna Niguel, Calif.) and to A 63-year-old woman presented with buccal alveolar palpation. The premolar severe pain and extraoral facial swelling in The articles for this was not tender to bite or palpation. The the upper left quadrant, which had begun month’s “Clinical cuspid did not respond to cold tests, the day before the visit and was wors- Showcase” section were whereas the premolar was hyperrespon- ening. Her medical history was noncon- written by speakers sive but with nonlingering pain consistent at the 2006 CDA Annual tributory except for mitral valve prolapse with reversible pulpitis. -

Oral Diagnosis: the Clinician's Guide
Wright An imprint of Elsevier Science Limited Robert Stevenson House, 1-3 Baxter's Place, Leith Walk, Edinburgh EH I 3AF First published :WOO Reprinted 2002. 238 7X69. fax: (+ 1) 215 238 2239, e-mail: [email protected]. You may also complete your request on-line via the Elsevier Science homepage (http://www.elsevier.com). by selecting'Customer Support' and then 'Obtaining Permissions·. British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress ISBN 0 7236 1040 I _ your source for books. journals and multimedia in the health sciences www.elsevierhealth.com Composition by Scribe Design, Gillingham, Kent Printed and bound in China Contents Preface vii Acknowledgements ix 1 The challenge of diagnosis 1 2 The history 4 3 Examination 11 4 Diagnostic tests 33 5 Pain of dental origin 71 6 Pain of non-dental origin 99 7 Trauma 124 8 Infection 140 9 Cysts 160 10 Ulcers 185 11 White patches 210 12 Bumps, lumps and swellings 226 13 Oral changes in systemic disease 263 14 Oral consequences of medication 290 Index 299 Preface The foundation of any form of successful treatment is accurate diagnosis. Though scientifically based, dentistry is also an art. This is evident in the provision of operative dental care and also in the diagnosis of oral and dental diseases. While diagnostic skills will be developed and enhanced by experience, it is essential that every prospective dentist is taught how to develop a structured and comprehensive approach to oral diagnosis. -

June 18, 2013 8:30 Am – 11:30 Am
Tuesday – June 18, 2013 8:30 am – 11:30 am Poster Abstracts – Tuesday, June 18, 2013 #1 ORAL LESIONS AS THE PRESENTING MANIFESTATION OF CROHN'S DISEASE V Woo, E Herschaft, J Wang U of Nevada, Las Vegas Crohn’s disease (CD) is an immune-mediated disorder of the gastrointestinal tract which together with ulcerative colitis, comprise the two major types of inflammatory bowel disease (IBD). The underlying etiology has been attributed to defects in mucosal immunity and the intestinal epithelial barrier in a genetically susceptible host, resulting in an inappropriate inflammatory response to intestinal microbes. The lesions of CD can affect any region of the alimentary tract as well as extraintestinal sites such as the skin, joints and eyes. The most common presenting symptoms are periumbilical pain and diarrhea associated with fevers, malaise and anemia. Oral involvement has been termed oral CD and may manifest as lip swelling, cobblestoned mucosa, mucogingivitis and linear ulcerations and fissures. Oral lesions may precede gastrointestinal involvement and can serve as early markers of CD. We describe a 6-year-old male who presented for evaluation of multifocal gingival erythema and swellings. His medical history was unremarkable for gastrointestinal disorders or distress. Histopathologic examination showed multiple well-formed granulomas that were negative for special stains and foreign body material. A diagnosis of granulomatous gingivitis was rendered. The patient was advised to seek consultation with a pediatric gastroenterologist and following colonoscopy, was diagnosed with early stage CD. Timely recognition of the oral manifestations of CD is critical because only a minority of patients will continue to exhibit CD-specific oral lesions at follow-up. -

Management of Dental Trauma in a Primary Care Setting Abstract
Guidance for the Clinician in Rendering Pediatric Care CLINICAL REPORT Management of Dental Trauma in a Primary Care Setting Martha Ann Keels, DDS, PhD, and THE SECTION ON ORAL abstract HEALTH The American Academy of Pediatrics and its Section on Oral Health have KEY WORDS developed this clinical report for pediatricians and primary care physi- dental trauma, dental injury, tooth, teeth, dentist, pediatrician cians regarding the diagnosis, evaluation, and management of dental ABBREVIATION trauma in children aged 1 to 21 years. This report was developed CT—computed tomography through a comprehensive search and analysis of the medical and den- This document is copyrighted and is property of the American tal literature and expert consensus. Guidelines published and updated Academy of Pediatrics and its Board of Directors. All authors have filed conflict of interest statements with the American by the International Association of Dental Traumatology (www.dental- Academy of Pediatrics. Any conflicts have been resolved through traumaguide.com) are an excellent resource for both dental and non- a process approved by the Board of Directors. The American dental health care providers. Pediatrics 2014;133:e466–e476 Academy of Pediatrics has neither solicited nor accepted any commercial involvement in the development of the content of this publication. The guidance in this report does not indicate an exclusive INTRODUCTION course of treatment or serve as a standard of medical care. Variations, taking into account individual circumstances, may be By 14 years of age, 30% of children have experienced a dental injury.1 appropriate. Many of these children are taken directly to their medical home, an urgent care center, or an emergency department for evaluation and treatment. -

Saving Smiles Avulsion Pathway (Page 20) Saving Smiles: Fractures and Displacements (Page 22)
Greater Manchester Local Dental Network SavingSmiles Improving outcomes following dental trauma First Edition I Spring 2017 Practitioners’ Toolkit Contents 04 Introduction to the toolkit from the GM Trauma Network 06 History & examination 10 Maxillo-facial considerations 12 Classification of dento-alveolar injuries 16 The paediatric patient 18 Splinting 20 The AVULSED Tooth 22 The BROKEN Tooth 23 Managing injuries with delayed presentation SavingSmiles 24 Follow up Improving outcomes 26 Long term consequences following dental trauma 28 Armamentarium 29 When to refer 30 Non-accidental injury 31 What should I do if I suspect dental neglect or abuse? 34 www.dentaltrauma.co.uk 35 Additional reference material 36 Dental trauma history sheet 38 Avulsion pathways 39 Fractues and displacement pathway 40 Fractures and displacements in the primary dentition 41 Acknowledgements SavingSmiles Improving outcomes following dental trauma Ambition for Greater Manchester Introduction to the Toolkit from The GM Trauma Network wish to work with our colleagues to ensure that: the GM Trauma Network • All clinicians in GM have the confidence and knowledge to provide a timely and effective first line response to dental trauma. • All clinicians are aware of the need for close monitoring of patients following trauma, and when to refer. The Greater Manchester Local Dental Network (GM LDN) has established a ‘Trauma Network’ sub-group. The • All settings have the equipment described within the ‘armamentarium’ section of this booklet to support optimal treatment. Trauma Network was established to support a safer, faster, better first response to dental trauma and follow up care across GM. The group includes members representing general dental practitioners, commissioners, To support GM practitioners in achieving this ambition, we will be working with Health Education England to provide training days and specialists in restorative and paediatric dentistry, and dental public health. -

Journal 2017
Journal of ENT masterclass ISSN 2047-959X Journal of ENT MASTERCLASS® Year Book 2017 Volume 10 Number 1 YEAR BOOK 2017 VOLUME 10 NUMBER 1 JOURNAL OF ENT MASTERCLASS® Volume 10 Issue 1 December 2017 Contents Free Courses for Trainees, Consultants, SAS grades, GPs & Nurses Welcome Message 3 CALENDER OF FREE RESOURCES 2018-19 Hesham Saleh Increased seats for specialist registrars & exam candidates ENT aspects of cystic fibrosis management 4 Gary J Connett ® 15th Annual International ENT Masterclass Paediatric swallowing disorders 8 Venue: Doncaster Royal Infirmary, 25-27th January 2019 Hayley Herbert and Shyan Vijayasekaran Special viva sessions for exam candidates Paediatric tongue-tie 14 Steven Frampton, Ciba Paul, Andrea Burgess and Hasnaa Ismail-Koch rd ® 3 ENT Masterclass China Paediatric oesophageal foreign bodies 20 Beijing, China, 12-13th May 2018 Emily Lowe, Jessica Chapman, Ori Ron and Michael Stanton Biofilms in paediatric otorhinolaryngology 26 3rd ENT Masterclass® Europe S Goldie, H Ismail-Koch, P.G. Harries and R J Salib Berlin, Germany, 14-15th Sept 2018 Intracranial complications of ear, nose and throat infections in childhood 34 Alice Lording, Sanjay Patel and Andrea Whitney ® ENT Masterclass Switzerland The superior canal dehiscence syndrome 41 Lausanne, 5-6th Oct 2018 Simon Richard Mackenzie Freeman Tympanosclerosis 46 ® ENT Masterclass Sri Lanka Priya Achar and Harry Powell Colombo, 16-17th Nov 2018 Endoscopic ear surgery 49 Carolina Wuesthoff, Nicholas Jufas and Nirmal Patel o Limited places, on first come basis. Early applications advised. o Masterclass lectures, Panel discussions, Clinical Grand Rounds Vestibular function testing 57 o Oncology, Plastics, Pathology, Radiology, Audiology, Medico-legal Karen Lindley and Charlie Huins Auditory brainstem implantation 63 Website: www.entmasterclass.com Harry R F Powell and Shakeel S Saeed CYBER TEXTBOOK on operative surgery, Journal of ENT Masterclass®, Surgical management of temporal bone meningo-encephalocoele and CSF leaks 69 Application forms Mr. -

Cracked Tooth Syndrome, an Update
International Journal of Applied Dental Sciences 2021; 7(2): 314-317 ISSN Print: 2394-7489 ISSN Online: 2394-7497 IJADS 2021; 7(2): 314-317 Cracked tooth syndrome, an update © 2021 IJADS www.oraljournal.com Received: 19-02-2021 Dariela Isabel Gonzalez-Guajardo, Guadalupe Magdalena Ramirez- Accepted: 21-03-2021 Herrera, Alejandro Mas-Enriquez, Guadalupe Rosalia Capetillo- Dariela Isabel Gonzalez-Guajardo Hernandez, Leticia Tiburcio-Morteo, Claudio Cabral-Romero, Rene Master in Sciences Student, Hernandez-Delgadillo and Juan Manuel Solis-Soto Universidad Autonoma de Nuevo Leon, Facultad de Odontologia, Monterrey, Nuevo Leon, CP 64460, DOI: https://doi.org/10.22271/oral.2021.v7.i2e.1226 Mexico Guadalupe Magdalena Ramirez- Abstract Herrera Introduction: Cracked tooth syndrome is defined as an incomplete fracture initiated from the crown and Professor, Universidad Autonoma de extending cervically, and sometimes gingivally, and is usually directed mesiodistally. Objective: To Nuevo Leon, Facultad de analyze the literature about cracked tooth syndrome, its etiology, prevalence, pulp involvement and Odontologia, Monterrey, Nuevo Leon, CP 64460, Mexico treatment. Methodology: Using the keywords “cracked tooth syndrome”, “etiology”, “prevalence”, “pulp Alejandro Mas-Enriquez involvement” and “treatment”, the MEDLINE/PubMed and ScienceDirect databases were searched, with Associate Professor, Universidad emphasis on the last 5 years. It was evaluated with the PRISMA and AMSTAR-2 guidelines. Autonoma de Nuevo Leon, Facultad de Odontologia, Monterrey, Nuevo Results: There are many causes for cracks, the main one being malocclusion. Another is due to Leon, CP 64460, Mexico restorations, pieces to which amalgam was placed due to the extension of the cavity for the retentions. The second lower molar presents more frequently fissures due to premature contact. -

Non-Surgical Management of Large Periapical Cyst Like Lesion: Case Report and Litterature Review
Open Access Journal of Oral Health and Dental Science Case Report ISSN: 2577-1485 Non-Surgical Management of Large Periapical Cyst Like Lesion: Case Report and Litterature Review Hammouti J1*, Chhoul H2 and Ramdi H2 1Resident, Faculty of Dental Medicine, Mohammed V University, Morocco 2Professor of Higher Education, Faculty of Dental Medicine, Mohammed V University, Morocco *Corresponding author: Hammouti J, Resident, Faculty of Dental Medicine, Dental Consultation and Treatment Center, Allal el Fassi Avenue, Mohammed Jazouli Street, Al Irfane City, BP 6212, Rabat–Institutes, Morocco, Tel: 00212665930945, E-mail: [email protected] Citation: Hammouti J, Chhoul H, Ramdi H (2019) Non-Surgical Management of Large Periapical Cyst Like Lesion: Case Report and Litterature Review. J Oral Health Dent Sci 3: 202 Article history: Received: 30 April 2019, Accepted: 21 May 2019, Published: 24 May 2019 Abstract This case report describes the non-surgical management of a large cyst-like periapical lesion in the mandible of an 11-year-old child with the chief complaint of periodic swelling from the mandibular anterior region with a history of traumatic accident in this area. Both mandibular left central and lateral incisors had enamel-dentin fracture. Root canals of these teeth were filled with calcium hydroxide. After 6 weeks, endodontic therapy was carried out on both teeth. Clinical and radiographic monitoring at 3 months revealed progressing bone healing. Complete periapical healing was observed at the 12 month recall. This report confirms that for management of a large periapical lesion the non-surgical procedure is essential and it can lead to complete healing of large lesions without invasive surgical treatments. -
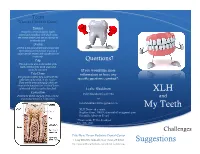
Teeth What Do I Need to Know?
Teeth What do I Need to Know? Enamel Enamel is a semitranslucent, highly mineralized crystalline solid which covers the crowns of teeth and acts as a barrier to protect the teeth. Dentin Dentin is less mineralized than enamel, but more mineralized than bone; it acts as a cushion for the enamel and a further barrier to the pulp. Pulp Questions? The pulp is the area in the middle of the tooth containing the blood vessels and nerves for that tooth. If you would like more Pulp Horns information or have any The projections of the pulp underneath the taller parts of the tooth, or the “cusps.” specific questions, contact*: These are the areas of the pulp which are closest to the functional (or “occlusal”) part of the tooth which is used to chew food. Leslie Blackburn Cementum XLH [email protected] Protects the dentin and pulp of the roots the and way enamel protects it in the crown. or [email protected] My Teeth XLH Network contact: Raghbir Kaur, DMD; [email protected] Scientific Advisory Board *Please include XLH in the subject line of the email. Challenges Yale-New Haven Pediatric Dental Center 1 Long Wharf Dr, Suite 403, New Haven, CT 06510 Suggestions http://www.ynhh.org/medical-services/dental_pediatric.aspx What is the Most Important Thing to Know? It is not your fault. People with XLH have unique dental challenges. Sometimes even when you are doing everything right you may still have dental problems. While it is important to do everything you can to keep your mouth healthy, it is also important to remember that you some things about your oral health are out of your control.