Full Text (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Universidade Federal De Juiz De Fora Pós-Graduação Em Ciências Biológicas Mestrado Em Comportamento E Biologia Animal
UNIVERSIDADE FEDERAL DE JUIZ DE FORA PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS MESTRADO EM COMPORTAMENTO E BIOLOGIA ANIMAL Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Juiz de Fora 2019 Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Dissertação apresentada ao Programa de Pós-Graduação em Ciências Biológicas, área de concentração: Comportamento e Biologia Animal da Universidade Federal de Juiz de Fora, como requisito parcial para obtenção do título de Mestre. Orientadora: Prof.ª. Drª. Sthefane D’ávila Juiz de Fora 2019 A todos que estiveram ao meu lado me apoiando e incentivando diante das dificuldades da carreira acadêmica, e incentivaram minha formação pessoal, profissional e dando-me suporte emocional. A vocês o meu eterno agradecimento! AGRADECIMENTOS Agradeço primeiramente a Deus por abençoar o meu caminho durante esse trabalho. A fé que tenho em Ti alimentou meu foco, minha força e minha disciplina. Depois aos meus amigos da Ciências Biológicas: Alexssandra Silva, Flávio Macanha, Isabel Macedo, Sue-helen Mondaini, Tayrine Carvalho, Kássia Malta e Yuri Carvalho meu eterno agradecimento, pois fizeram uma contribuição valiosa para a minha jornada acadêmica com seus conselhos, auxílio, palavras de apoio e risadas. Também agradeço a todos aqueles amigos que de forma direta ou indireta estiveram ajudando e torcendo por mim, em especial a Ana Claudia Mazetto, Ana Clara Files, Tamires Lima, Lígia Araújo, Raquel Seixas, Natália Corrêa e Carlota Augusta. Vocês foram fundamentais para minha formação. Agradeço à minha orientadora Sthefane D' ávila, que acompanhou meu percurso ao longo dos últimos anos e ofereceu uma orientação repleta de conhecimento, sabedoria e paciência. -

Veronicella Spp.*
Veronicella spp.* *In April 2013, the family Veronicellidae, a target on the 2013 and 2014 AHP Prioritized Pest Lists, was broken down into six genera of concern, including Veronicella spp. Information in the datasheet may be at the family, genus, or species level. Information for specific species within the genus is included when known and relevant; other species may occur in the genus and are still reportable at the genus level. Portions of this document were taken Figure 1. Veronicella cubensis (Pfeiffer), (Image directly from the New Pest Response courtesy of David Robinson, USDA-APHIS-PPQ) Guidelines for Tropical Terrestrial Gastropods (USDA-APHIS, 2010a). Scientific Names Veronicella cubensis (Pfeiffer, 1840) Veronicella sloanii (Cuvier, 1817) Synonyms: Veronicella cubensis Onchidium cubense Pfeiffer, 1840, Onchidium cubensis, Veronicella cubensis Thomé [Thomé], 1975 Veronicella sloanei Vaginulus sloanei Férussac, [Férussac] Vaginulus laevis de Blainville, 1817 Common Name No common name, leatherleaf slugs Figure 2. Veronicella sloanei (Cuvier), (Image courtesy of David Robinson, USDA-APHIS-PPQ) Veronicella cubensis: Cuban slug Veronicella sloanii: Pancake slug Type of Pest Mollusk Taxonomic Position Class: Gastropoda, Order: Systellommatophora, Family: Veronicellidae Last update: May 2014 1 Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List for FY 2011 – 2015* *Originally listed under the family Veronicellidae. Pest Description Veronicellidae are anatomically distinct from many other terrestrial slugs in that they have a posterior anus, eyes on contractile tentacles, and no pulmonate lung. The sensory tentacles are bilobed. This family also lacks a mantel cavity (Runham and Hunter, 1970). Although this family is fairly easy to tell apart from others, species within this family can be difficult to distinguish due to similar morphology between species and multiple color variations within a single species. -

Gastropoda: Stylommatophora)1 John L
EENY-494 Terrestrial Slugs of Florida (Gastropoda: Stylommatophora)1 John L. Capinera2 Introduction Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discus- sion of the situation in Florida, including problems with Figure 1. Lateral view of slug showing the breathing pore (pneumostome) open. When closed, the pore can be difficult to locate. slug identification and taxonomy, as well as the behavior, Note that there are two pairs of tentacles, with the larger, upper pair ecology, and management of slugs. bearing visual organs. Credits: Lyle J. Buss, UF/IFAS Biology as nocturnal activity and dwelling mostly in sheltered Slugs are snails without a visible shell (some have an environments. Slugs also reduce water loss by opening their internal shell and a few have a greatly reduced external breathing pore (pneumostome) only periodically instead of shell). The slug life-form (with a reduced or invisible shell) having it open continuously. Slugs produce mucus (slime), has evolved a number of times in different snail families, which allows them to adhere to the substrate and provides but this shell-free body form has imparted similar behavior some protection against abrasion, but some mucus also and physiology in all species of slugs. -

Malacofauna En Dos Sistemas Silvopastoriles En Estelí, Nicaragua
Malacofauna en dos sistemas silvopastoriles en Estelí, Nicaragua Malacofauna in two silvopastoral systems in Estelí, Nicaragua González-Valdivia Noel Antonio1, Martínez-Puc Jesús Froylán1*, Echavarría-Góngora Elías de Jesus2 Datos del Artículo Resumen 1Departamento de Ingenierías, Instituto Tecnológico de Chiná, Tecnológico La diversidad malacológica en dos sistemas silvopastoriles tradicionales, uno en bosque seco (Las Mesitas) y otro en Nacional de México. Calle 8, entre 22 y 28, Chiná, Campeche, México. CP. bosque de pino-encino (Picacho-Tomabú), ubicados en el municipio de Estelí, Nicaragua fue determinada con el fin de 25420. establecer comparaciones entre los ensambles de moluscos terrestres entre ambos sistemas. Para esto, durante la estación [email protected] 2Departamento de Ciencias Económico lluviosa (mayo a octubre) se realizaron muestreos una vez establecidas las lluvias (agosto a septiembre), siguiendo el Administrativas, Instituto Tecnológico de 2 Chiná, Tecnológico Nacional de México. método de conteo directo en 20 parcelas de 2.25 m y 20 árboles seleccionados al azar para cada localidad. Los moluscos Calle 8, entre 22 y 28, Chiná, Campeche, México. CP. 25420. extraídos de las muestras fueron identificados en el Centro Malacológico de la Universidad Centro Americana (UCACM), en Managua. Se estimaron los índices de riqueza, diversidad, equidad, dominancia y abundancia de especies *Dirección de contacto: para cada sistema y la asociación existente entre estos. Se determinó la presencia de un total de 47 especies, 41 de ellas Departamento de Ingenierías, Instituto Tecnológico de Chiná, Tecnológico presentes en Las Mesitas y 22 en el Picacho-Tomabú, incluyendo las 16 especies comunes entre ambas localidades Nacional de México. Calle 8, entre 22 y 28, Chiná, Campeche, México. -

Slugs (Of Florida) (Gastropoda: Pulmonata)1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-087 Slugs (of Florida) (Gastropoda: Pulmonata)1 Lionel A. Stange and Jane E. Deisler2 Introduction washed under running water to remove excess mucus before placing in preservative. Notes on the color of Florida has a depauparate slug fauna, having the mucus secreted by the living slug would be only three native species which belong to three helpful in identification. different families. Eleven species of exotic slugs have been intercepted by USDA and DPI quarantine Biology inspectors, but only one is known to be established. Some of these, such as the gray garden slug Slugs are hermaphroditic, but often the sperm (Deroceras reticulatum Müller), spotted garden slug and ova in the gonads mature at different times (Limax maximus L.), and tawny garden slug (Limax (leading to male and female phases). Slugs flavus L.), are very destructive garden and greenhouse commonly cross fertilize and may have elaborate pests. Therefore, constant vigilance is needed to courtship dances (Karlin and Bacon 1961). They lay prevent their establishment. Some veronicellid slugs gelatinous eggs in clusters that usually average 20 to are becoming more widely distributed (Dundee 30 on the soil in concealed and moist locations. Eggs 1977). The Brazilian Veronicella ameghini are round to oval, usually colorless, and sometimes (Gambetta) has been found at several Florida have irregular rows of calcium particles which are localities (Dundee 1974). This velvety black slug absorbed by the embryo to form the internal shell should be looked for under boards and debris in (Karlin and Naegele 1958). -

4. Distribution Patterns of Amphibians in the West Indies
- Pp. 2 1 1-254 In, Duellman, W. E. (Ed.) Patterns of distribution of amphibians: A global perspective. The Johns Hopkins University Press, Baltimore. (I~w) 4. Distribution Patterns of Amphibians in the West Indies Department of Biology 208 Mueller Laboratory, Pennsylvania State University University Park Pennsylvania 16802, USA ABSTRACT There are 174 species of amphibians known from the West Indies, 167 of these are native and eight introduced; 164 (98%) of the native species are endemic. The native fauna, all anurans, belongs to four families: Bufonidae (1 genus, 11 species), Dendrobatidae (1, I), Hylidae (4, 11), and Leptodactylidae (2, 144). Most species (84%) of West Indian amphibians belong to the large leptodactylid genus Eleutherodactylus. The greatest diversity of bufonids (8 species) occurs in Cuba, and of hylids (5 species) in Jamaica. Except for two Cuban species occurring elsewhere, single-island endemism is 100% in the Greater Antilles, and most species are restricted to small areas (c 100 km2) within an island, and 11 species (7%) are known from only type-localities. There are 50 native species (96% endemic) in Cuba, 22 species (100% endemic) in Jamaica, 63 species (100% endemic) in Hispaniola, 20 species (100% endemic) in the Puerto Rican Bank, and 10 species (90% endemic) in the Lesser Antilles. Only two species are native to the Bahamas Bank, and one species is native to the Cayman Islands; none is endemic. Ten percent of the amphibian fauna, including a new family for the West lndies, has been discovered in the last four years, this rate of discovery suggests that our knowiedge of species diversity is far from complete. -

2019 Journal Publications
2019 Journal Publications January Ayala, C. Ramos, A. Merlo, Á. Zambrano, L. (2019). Microhabitat selection of axolotls, Ambystoma mexicanum , in artificial and natural aquatic systems. Hydrobiologia, 828(1), pp.11-20. https://link.springer.com/article/10.1007/s10750-018-3792-8 Bélouard, N. Petit, E. J. Huteau, D. Oger, A. Paillisson, J-M. (2019). Fins are relevant non-lethal surrogates for muscle to measure stable isotopes in amphibians. Knowledge & Management of Aquatic Ecosystems, 420. https://www.kmae-journal.org/articles/kmae/pdf/2019/01/kmae180087.pdf Bignotte-Giró, I. Fong G, A. López-Iborra, G. M. (2019). Acoustic niche partitioning in five Cuban frogs of the genus Eleutherodactylus. Amphibia Reptilia,(40)1. https://brill.com/abstract/journals/amre/40/1/article-p1_1.xml Boissinot, A. Besnard, A. Lourdais, O. (2019). Amphibian diversity in farmlands: Combined influences of breeding-site and landscape attributes in western France. Agriculture, Ecosystems & Environment 269, pp.51-61. https://www.sciencedirect.com/science/article/pii/S0167880918303979 Borges, R. E. de Souza Santos, L. R. Assis, R. A. Benvindo-Souza, M. (2019). Monitoring the morphological integrity of neotropical anurans. Environmental Science and Pollution Research, 26(3), pp. 2623–2634. https://link.springer.com/article/10.1007/s11356-018-3779-z Borteiro, C. Kolenc, F. Verdes, J. M. Debat, C. M. Ubilla, M. (2019). Sensitivity of histology for the detection of the amphibian chytrid fungus Batrachochytrium dendrobatidis. Journal of Veterinary Diagnostic Investigation, 01/19/2019, p.104063871881611 https://journals.sagepub.com/doi/abs/10.1177/1040638718816116 Bozzuto, C. Canessa, S. (2019). Impact of seasonal cycles on host-pathogen dynamics and disease mitigation for Batrachochytrium salamandrivorans. -

In Vitro Production and Biocontrol Potential of Nematodes Associated with Molluscs
In vitro production and biocontrol potential of nematodes associated with molluscs by Annika Pieterse Dissertation presented for the degree of Doctor of Nematology in the Faculty of AgriSciences at Stellenbosch University Co-supervisor: Professor Antoinette Paula Malan Co-supervisor: Doctor Jenna Louise Ross March 2020 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. This dissertation includes one original paper published in a peer-reviewed journal. The development and writing of the paper was the principal responsibility of myself and, for each of the cases where this is not the case, a declaration is included in the dissertation indicating the nature and extent of the contributions of co-authors. March 2020 Copyright © 2020 Stellenbosch University All rights reserved II Stellenbosch University https://scholar.sun.ac.za Acknowledgements First and foremost, I would like to thank my two supervisors, Prof Antoinette Malan and Dr Jenna Ross. This thesis would not have been possible without their help, patience and expertise. I am grateful for the opportunity to have been part of this novel work in South Africa. I would like to thank Prof. Des Conlong for welcoming me at SASRI in KwaZulu-Natal and organizing slug collections with local growers, as well as Sheila Storey for helping me transport the slugs from KZN. -
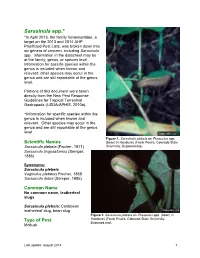
Sarasinula Spp.*
Sarasinula spp.* *In April 2013, the family Veronicellidae, a target on the 2013 and 2014 AHP Prioritized Pest Lists, was broken down into six genera of concern, including Sarasinula spp. Information in the datasheet may be at the family, genus, or species level. Information for specific species within the genus is included when known and relevant; other species may occur in the genus and are still reportable at the genus level. Portions of this document were taken directly from the New Pest Response Guidelines for Tropical Terrestrial Gastropods (USDA-APHIS, 2010a). *Information for specific species within the genus is included when known and relevant. Other species may occur in the genus and are still reportable at the genus level. Figure 1. Sarasinula plebeia on Phaseolus spp. Scientific Names (bean) in Honduras (Frank Peairs, Colorado State Sarasinula plebeia (Fischer, 1871) University, Bugwood.org). Sarasinula linguaeformis (Semper, 1885) Synonyms: Sarasinula plebeia Vaginulus plebeius Fischer, 1868 Sarasinula dubia (Semper, 1885) Common Name No common name, leatherleaf slugs Sarasinula plebeia: Caribbean leatherleaf slug, bean slug Figure 2. Sarasinula plebeia on Phaseolus spp. (bean) in Honduras (Frank Peairs, Colorado State University, Type of Pest Bugwood.org). Mollusk Last update: August 2014 1 Taxonomic Position Class: Gastropoda, Order: Systellommatophora, Family: Veronicellidae Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List for FY 2011 – 2015* *Originally listed under the family Veronicellidae. Pest Description Veronicellidae are anatomically distinct from many other terrestrial slugs in that they have a posterior anus, eyes on contractile tentacles, and no pulmonate lung. The sensory tentacles are bilobed. This family also lacks a mantel cavity (Runham and Hunter, 1970). -

Herpetological Information Service No
Checklist and Bibliograpy OF Cuban Amphibians (Anura) Vilma Rivalta Gonzalez Institute de Ecologia y Sistematica Ministerio de Ciencia, Tecnologia y Medio Ambiente SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 124 2000 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. Introduction The amphibian fauna of Cuba consists of 55 species described to date, of which 52 (94.5 %) are endemic. It contains a total of 67 taxa, including subspecies, nine of which have been described since the most recently published checklist (Powell et al., 1996). Herein, I list all new and previously described species of amphibians. This bibliography of the Cuban amphibians deals, in a great part, with taxonomy and geographical distribution. There are many papers in which the species of a given region are listed, and some data about their habitats are presented. -

Snail and Slug Dissection Tutorial: Many Terrestrial Gastropods Cannot Be
IDENTIFICATION OF AGRICULTURALLY IMPORTANT MOLLUSCS TO THE U.S. AND OBSERVATIONS ON SELECT FLORIDA SPECIES By JODI WHITE-MCLEAN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2012 1 © 2012 Jodi White-McLean 2 To my wonderful husband Steve whose love and support helped me to complete this work. I also dedicate this work to my beautiful daughter Sidni who remains the sunshine in my life. 3 ACKNOWLEDGMENTS I would like to express my sincere gratitude to my committee chairman, Dr. John Capinera for his endless support and guidance. His invaluable effort to encourage critical thinking is greatly appreciated. I would also like to thank my supervisory committee (Dr. Amanda Hodges, Dr. Catharine Mannion, Dr. Gustav Paulay and John Slapcinsky) for their guidance in completing this work. I would like to thank Terrence Walters, Matthew Trice and Amanda Redford form the United States Department of Agriculture - Animal and Plant Health Inspection Service - Plant Protection and Quarantine (USDA-APHIS-PPQ) for providing me with financial and technical assistance. This degree would not have been possible without their help. I also would like to thank John Slapcinsky and the staff as the Florida Museum of Natural History for making their collections and services available and accessible. I also would like to thank Dr. Jennifer Gillett-Kaufman for her assistance in the collection of the fungi used in this dissertation. I am truly grateful for the time that both Dr. Gillett-Kaufman and Dr. -

Molecular and Morphological Data Support Recognition of a New Genus of New World Direct-Developing Frog (Anura: Terrarana) From
Zootaxa 3986 (2): 151–172 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3986.2.1 http://zoobank.org/urn:lsid:zoobank.org:pub:82BDF224-FE83-4792-9AD1-D954B96B1136 Molecular and morphological data support recognition of a new genus of New World direct-developing frog (Anura: Terrarana) from an under-sampled region of South America MATTHEW P. HEINICKE1,4, CÉSAR L. BARRIO-AMORÓS2 & S. BLAIR HEDGES3 1Department of Natural Sciences, University of Michigan-Dearborn, 4901 Evergreen Road, Dearborn, MI 48128 USA 2Doc Frog Expeditions, Apartado Postal 220-8000, San José, Pérez Zeledón, San Isidro del General, 11901 Costa Rica. E-mail: [email protected] 3Center for Biodiversity, College of Science and Technology, Temple University, Philadelphia, PA 19122. E-mail: [email protected] 4Corresponding author. E-mail: [email protected] Abstract We describe a new genus of New World direct-developing frog (Terrarana) from the northern Andes of Venezuela and adjacent Colombia. Tachiramantis gen. nov. includes three species formerly placed in the large genus Pristimantis. Mo- lecular phylogenetic analysis of data from five nuclear and mitochondrial genes shows that Tachiramantis is not part of Pristimantis or any other named genus in its family (Craugastoridae or Strabomantidae). Morphological evidence further supports the distinctiveness of Tachiramantis, which has several aspects of skull morphology that are rare or absent in Pristimantis and synapomorphic for Tachiramantis, including frontoparietal-prootic fusion and degree of vomer develop- ment. The terminal phalanges, which narrow greatly before expanding at the tips, may represent an additional morpholog- ical synapomorphy.