ARUP Specimen Transport Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

12.1 12. Pressure Vessels: Combined Stresses Cylindrical Or Spherical
12. Pressure Vessels: Combined Stresses Cylindrical or spherical pressure vessels (e.g., hydraulic cylinders, gun barrels, pipes, boilers and tanks) are commonly used in industry to carry both liquid s and gases under pressure. When the pressure vessel is exposed to this pressure, the material comprising the vessel is subjected to pressure loading, and hence stresses, from all directions. The normal stresses resulting from this pressure are functions of the radius of the element under consideration, the shape of the pressure vessel (i.e., open ended cylinder, closed end cylinder, or sphere) as well as the applied pressure. Two types of analysis are commonly applied to pressure vessels. The most common method is based on a simple mechanics approach and is applicable to “thin wall” pressure vessels which by definition have a ratio of inner radius, r, to wall thickness, t, of r/t≥10. The second method is based on elasticity solution and is always applicable regardless of the r/t ratio and can be referred to as the solution for “thick wall” pressure vessels. Both types of analysis are discussed here, although for most engineering applications, the thin wall pressure vessel can be used. Thin-Walled Pressure Vessels Several assumptions are made in this method. 1) Plane sections remain plane 2) r/t ≥ 10 with t being uniform and constant 3) The applied pressure, p, is the gage pressure (note that p is the difference between the absolute pressure and the atmospheric pressure) 4) Material is linear-elastic, isotropic and homogeneous. 5) Stress distributions throughout the wall thickness will not vary 6) Element of interest is remote from the end of the cylinder and other geometric discontinuities. -

Coalescing Filters - to 175 Psig @ -20 to 200°F Series R20- Enameled Carbon Steel ◊ Series R22- 304 Stainless • Intake Air Flows to 40,000 SCFM Std
click here to return to website Coalescing Filters - to 175 psig @ -20 to 200°F Series R20- Enameled Carbon Steel ◊ Series R22- 304 Stainless • Intake Air Flows to 40,000 SCFM Std. • ASME U Stamp Std., Nat’l. Board Registered • Exceptionally Low ∆P, High Flow • Pleated Element Design - Exceptional Useful Filter Area • Hinged Swing Bolt Closure, Easy Access, O Ring Seal • 304SS Throat Safety Cages and ∆P Taps Std. • Rugged Enameled Steel or 304SS Construction Series R20 coalescing filters are fabricated from rugged enameled carbon steel, designed, constructed in accordance w/ASME Boiler & Pressure Vessel Code requirements for unfired pressure vessels. Any model can be modified to fit your needs. • Standard Connection Sizes from 1" to 12" NPT or raised face flange in-line connections are std. Alt. connections and/or an elevated discharge are avail- able. A hinged swing bolt closure is standard on models R20-0002 & larger. • Coalescing Filter Media. Sparks™ #907 media is composed of microfine borosilicate glass fibers bonded with phenolic resin. Together with a textile prefilter and a final drain layer, these pleated elements are remarkably effective at coalescing fine entrained oil and aqueous vapor mist from air/gas flows with very low ∆P. Experience has demonstrated high removal (over 90%) in dealing with 1.0 to 0.3µ aerosols. Other optional filter media such as #926 exceeds 95% removals. Individual performance will vary with the specific viscosity and vapor pressure of liquid con- taminates. • Options: Models R20-0202-RF-030 and larger include CS leg supports. (add 18" to OH) Carbon steel support legs in any length, gauges, and special finishes, are optional on any model. -

Design of Pressure Vessle (Air Bottle)
INTERNATIONAL JOURNAL FOR RESEARCH IN EMERGING SCIENCE AND TECHNOLOGY, VOLUME-4, ISSUE-1, JAN-2017 E-ISSN: 2349-7610 Design of Pressure Vessle (Air Bottle) N.V.Mahesh Babu.T1, Nersu Radhika2, Dr.P.Srinivasa Rao3 and Dr.B.Sudheer Prem Kumar4 1Associate Professor, Department of Mechanical Engineering, Guru Nanak Institutions Technical Campus, Ibrahimpatnam, Telangana 501 506, [email protected]. 2Assistant Professor, H & S Department, Sri Indu College of Engineering and Technology, Ibrahimpatnam, Telangana 501 506. 2 [email protected]. 3 Professor, Department of Mechanical Engineering,Al-Habeeb College of Engineering and Technology,Chevella, Telangana, [email protected] 4Professor & Chairman(Board of Studies) Mechanical Engineering, JNT University,Hyderabad, Telangana 500 085, [email protected], [email protected] ABSTRACT This is a paper that presents the design of a pressure vessel (Air Bottle). High pressure rise is developed in the pressure vessel and pressure vessel has to withstand severe forces. In the design of pressure vessel safety is the primary consideration, due the potential impact of possible accident. There have a few main factors to design the safe pressure vessel. This writing is focusing on analyzing the safety parameter for allowable working pressure. The cylinder is designed by considering the pressure, temperature and other constraints. Analysis of strength is made analytically and validation is done by ANSYS model and analysis. Keywords — Air bottle, ASME Code, Finite Element Analysis, ANSYS, Design for Fatigue. 1. INTRODUCTION 2. TYPE OF STRESS INDUCED IN VESSELS Pressure vessels are containers for containment of pressure, Generally there are two types of stresses induced. -

Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging
Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging Components of an aseptic processing system Aseptic processing is a thermal process in which the product and container are sterilized separately and brought together in a sterile environment. It involves pumping, deaeration, and sterilization of a food product, followed by holding it for a specified period of time (in a holding tube -- required to have a 1/4" rise per foot length of tube), cooling it, and finally packaging it in a sterile container. The use of high temperature for a short period of time (in comparison with conventional canning) in aseptic processing yields a high quality product. Care should be taken to ensure that all process calculations are performed after the deaeration stage and not based on the initial raw product. Deaeration is accomplished in a vessel maintained at a certain degree of vacuum by means of a vacuum pump. The product is fed into the vessel at 55 - 70 /C through a nozzle at the center of the vessel. Vacuum is controlled to obtain a product flash of about 5 /C. An internal spiral condenser condenses vapors and other condensable gases. The deaerated product is discharged through the bottom and pumped to the heating section. Another important part of an aseptic processing system is the back pressure valve which provides sufficient pressure to prevent boiling of the product at processing temperatures which can be as high as 125-130 /C. An aseptic surge tank provides the means for product to be continuously processed even if the packaging system is not operational due to any malfunction. -

Gas Generator Bottle Introduction SCIENTIFIC This Gas Generator Setup Provides an Easy Way to Generate and Collect Gas
Gas Generator Bottle Introduction SCIENTIFIC This gas generator setup provides an easy way to generate and collect gas. Specific instructions are provided for the generation of hydrogen gas using zinc and acid. Concepts • Generation of gases • Water displacement Materials Hydrochloric acid solution, HCl, 3 M Glass plates or Sulfuric acid solution, H2SO4, 3 M Glass tubing Mossy zinc, Zn, 6 g Pneumatic trough Water, tap Rubber tubing Bent glass tubing* Silicone grease packet* Gas collecting bottles or tubes, 3 or 4 Thistle tube* Gas generator bottle* Two-hole rubber stopper* *Materials included. Safety Precautions Hydrochloric acid solution is toxic by ingestion and inhalation and is severely corrosive to skin, eyes and other tissues, as is sulfuric acid solu- tion. Hydrogen gas is a highly flammable gas and a severe fire hazard. Exercise extreme caution when testing the gas and keep the gas generator away from flames. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. This activity requires the use of hazardous components and/or has the potential for hazardous reactions. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Procedure 1. Set up the apparatus as shown in the figure to the right. Lubricate the glass tubing and thistle tube with silicone grease before inserting into the stopper. Make sure Thistle tube the water level is above the platform. Prepare bottles for collecting gas by water Two-hole rubber stopper displacement. To do this, fill each gas collecting bottle (or tube) over the brim with tap water, and then cover each with a flat glass plate. -

Trace Elements Specimen Collection Guide
500 Chipeta Way Salt Lake City, Utah 84108-1221 Phone: 801-583-2787 | toll free: 800-242-2787 A nonprofit enterprise of the University of Utah and its Department of Pathology Fax: 801-522-2706 | aruplab.com TRACE ELEMENTS SPECIMEN COLLECTION GUIDE Analysis for trace elements at ARUP is performed in a clean laboratory environment that includes a system of positive- pressure and HEPA-filtered air. Since detection limits for many trace elements are calculated in parts per billion, this helps minimize environmental contamination of specimens. Additionally, contamination control during specimen collection provides more accurate and clinically useful results. Note: Refer to the ARUP Laboratory Test Directory for specific instructions when collecting specimens for cadmium exposure testing (http://ltd.aruplab.com/Tests/Pub/0025013). Urine Collection Tissue Although it is nearly impossible to obtain a completely Follow information on individual pages of the laboratory uncontaminated urine specimen, steps can be taken to test directory. minimize environmental contamination. Avoid collecting the specimen in an area where Whole Blood, Serum, and Red Blood Cell Collection Materials comprising the tube and the tube top are a environmental contamination is likely to occur. In an consideration. Rubber stoppers in collection tubes other industrial or construction setting, it is important that than the royal blue tube are known to contain elements clothing worn in the workplace be removed prior to that can contaminate a specimen. Contamination can be specimen collection to prevent dust on the clothing introduced as the needle punctures the stopper in a tube from contaminating the specimen. other than royal blue. Most of this also holds true for Wash and dry hands thoroughly prior to collection. -

Boiler and Pressure Vessel Code Or BPVC
2017 Boiler and Pressure Vessel Code AN INTERNATIONAL CODE GO.ASME.ORG/BPVC17 The American Society of Mechanical Engineers® (ASME®) FOR DETAILS, CALL 1-800-THE-ASME (1-800-843-2763) (OR) 1-973-882-1170 (OR) VISIT GO.ASME.ORG/BPVC17 BOILERS AND PRESSURE VESSELS Since its first issuance in 1914, ASME’s BPVC has pioneered modern standards-development, maintaining a commitment to enhance public safety and technological advancement to meet the needs of a changing world. This “International Historic Mechanical Engineering Landmark” now has been incorporated into the laws of state and local jurisdictions of the United States and nine Canadian provinces. The BPVC is in use in 100 countries ASME’S BOILER AND PRESSURE VESSEL CODE (BPVC) 2017 around the world, with translations into a number of languages. The boiler and pressure-vessel sections of the BPVC have long been considered essential within such industries as electric power-generation, petrochemical, and transportation, among others. NUCLEAR ASME has played a vital role in supporting the nuclear industry since its inception, when ASME codes, standards and conformity assessment programs, ASME issued its first Standard, Code for originally developed for fossil fuel-fired the Conduct of Trials of Steam Boilers, in plants, were applied to nuclear power- 1884. This paper evolved into Rules for the plant construction. The nuclear sections Construction of Stationary Boilers and for of the BPVC reflect the best-practices Allowable Working Pressure – the first of industry, while contributing to more edition of ASME’s now-legendary Boiler than a half-century of safety for the and Pressure Vessel Code (BPVC) – issued general public. -

Pressure Vessels BT Series Replaceable Bladder Expansion Tank with Bottom System Connection RDT Series Fixed Bladder Expansion Tank
SINCE 1981 Pressure Vessels BT Series Replaceable Bladder Expansion Tank with Bottom System Connection RDT Series Fixed Bladder Expansion Tank SEP Series Vortex - Tangential Air Separator ADSR/AD Series In-Line Air/Dirt Separator (With or Without Strainer) RLU/RWU Series Hot Water Storage Tank CBT Series Buffer Tank www.flofab.com 003-cat-2019-pv Tanks (1).indd 1 2019-03-29 18:19:06 TABLE OF CONTENT RDT EXPANSION TANKS...........................................................................................................................2 BT EXPANSION TANKS..............................................................................................................................3 BT & RDT EXPANSION TANKS................................................................................................................4 INSTALLATION OF TANKS.......................................................................................................................5 SEP VORTEX TANGENTIAL AIR SEPARATOR..............................................................................6-8 ADSR/AD & ADSF IN-LINE AIR/DIRT SEPARATOR.................................................................9-14 RLU HOT WATER STORAGE TANK...............................................................................................15-20 RWU HOT WATER STORAGE TANK..............................................................................................21-24 CBT BUFFER TANK............................................................................................................................25-26 -

Rigid Intermediate Bulk Containers: United States
Freedonia Focus Reports US Collection Rigid Intermediate Bulk Containers: United States February 2019 CLICK TO ORDER FULL REPORT BROCHURE www.freedoniafocusreports.com CLICK TO ORDER FULL REPORT Table of Contents 1. Highlights 3 2. Market Environment 4 Historical Trends 4 Key Economic Indicators 5 Competition from Reconditioned & Refurbished Packaging 6 Regulatory Factors 8 3. Segmentation & Forecasts 10 Products 10 Plastic Body 11 Metal Body 12 Other Rigid Intermediate Bulk Containers 13 Markets 14 Chemicals & Pharmaceuticals 15 Food & Beverages 15 Petroleum & Lubricants 16 Hazardous Waste 16 Agricultural & Horticultural Products 16 Plastic & Rubber 17 Other Markets 17 4. Industry Structure 19 Industry Characteristics 19 Market Leaders 20 Mauser Packaging Solutions 20 SCHÜTZ 21 Greif 21 5. About This Report 22 Scope 22 Sources 22 Industry Codes 23 Freedonia Methodology 23 Resources 25 Rigid Intermediate Bulk Containers: United States 1 ©2019 The Freedonia Group. All rights reserved. List of Tables & Figures Figure 1 | Key Trends in the US Rigid Intermediate Bulk Container Market, 2018 – 2023 3 Figure 2 | US Rigid Intermediate Bulk Container Demand Trends, 2008 – 2018 4 Table 1 | Key Indicators for US Rigid Intermediate Bulk Container Demand, 2008 – 2023 (US$ bil) 5 Figure 3 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Table 2 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Figure 4 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (%) 12 Figure 5 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Table 3 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Figure 6 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (%) 18 Table 4 | Leading Suppliers in the US Rigid Intermediate Bulk Container Market by Product 20 Table 5 | NAICS & SIC Codes Related to Rigid Intermediate Bulk Containers 23 Rigid Intermediate Bulk Containers: United States 2 ©2019 The Freedonia Group. -

Nalgene and Nunc Centrifuge Ware Catalog
Nalgene and Nunc Centrifuge Ware Select the right vessel and spin with confidence Spin with confidence at virtually any scale The process of selecting a centrifuge and rotor can feel like the easy part when faced with choosing the tube or bottle that is the right fit for both the rotor and application. There are several factors to consider when selecting the correct vessel for each application: • Chemical compatibility • Volume • Temperature • Relative centrifugal force (RCF) required • Protocols to be used for loading and sample recovery • Cleaning and autoclaving steps Understanding your requirements before selecting a tube or bottle ensures you make the right choice. Whether your application includes the need for separations, large volume pelleting, protein purification or DNA isolation, the comprehensive selection of Thermo Scientific™ Nalgene™ and Thermo Scientific™ Nunc™ centrifuge ware offers a solution for virtually scales and is available in sizes from 10 mL to 2 L. Such a broad offering means a tube or bottle for many spins – from clinical and bioproduction, to processing bacteria, yeast, tissue, and viruses. Contents Centrifuge tubes 4 Centrifuge bottles 20 Closures and adaptors 31 Resources 36 Simplify performance at every turn with a reliable and safe approach to centrifugation Nalgene Conical-Bottom Centrifuge Tubes polypropylene copolymer Thermo Scientific™ Nalgene™ PPCO conical-bottom centrifuge tubes with molded-in graduations have excellent chemical resistance. Designed for low-speed centrifugation in refrigerated and non-refrigerated centrifuges details • Translucent PPCO is compatible with a wide range of lab reagents • Conical bottoms concentrate pellet in a small area for easy isolation and retrieval • Molded-in graduations last the life of the tube • Last longer than polycarbonate tubes under conditions of repeated Note: Centrifuge tubes must be filled at least 80% for proper performance. -

Guidelines for Pressure Vessel Safety Assessment
11^^^^ United States Department of Commerce National Institute of Standards and Tectinology NIST Special Publication 780 Guidelines for Pressure Vessel Safety Assessment Sumio Yukawa NATIONAL INSTITUTE OF STANDARDS & TECHNOLOGY Research Information Center Gaithersburg, MD 20899 DATE DUE Demco. Inc. 38-293 NIST Special Publication 780 Guidelines for Pressure Vessel Safety Assessment Sumio Yukawa Materials Reliability Division Materials Science and Engineering Laboratory National Institute of Standards and Technology Boulder, CO 80303 Sponsored by Occupational Safety and Health Administration U.S. Department of Labor Washington, DC 20210 Issued April 1990 U.S. Department of Commerce Robert A. Mosbacher, Secretary National Institute of Standards and Technology John W. Lyons, Director National Institute of Standards U.S. Government Printing Office For sale by the Superintendent and Technology Washington: 1990 of Documents Special Publication 780 U.S. Government Printing Office Natl. Inst. Stand. Technol. Washington, DC 20402 Spec. Publ. 780 75 pages (Apr. 1990) CODEN: NSPUE2 CONTENTS Page ABSTRACT vii 1. INTRODUCTION 1 2. SCOPE AND GENERAL INFORMATION 1 2 . 1 Scope 1 2.2 General Considerations 3 3. PRESSURE VESSEL DESIGN 4 3.1 ASME Code 4 3.1.1 Section VIII of ASME Code 5 3.1.2 Scope of Section VIII 5 3.1.3 Summary of Design Rules and Margins 6 3.1.4 Implementation of ASME Code 9 3.2 API Standard 620 10 3.2.1 Scope of API 620 12 3.2.2 Design Rules 12 3.2.3 Implementation of API 620 12 3.3. Remarks on Design Codes 14 4. DETERIORATION AND FAILURE MODES 14 4.1 Preexisting Causes 14 4.1.1 Design and Construction Related Deficiencies. -
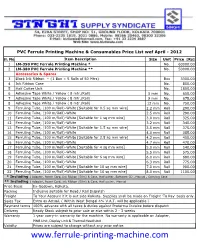
Ferrule Printing Machine & Consumables Price List Wef April - 2012
PVC Ferrule Printing Machine & Consumables Price List wef April - 2012 Sl. No. Item Description Size Unit Price [Rs] 1 LM-390 PVC Ferrule Printing Machine * No. 60000.00 2 LM-380 PVC Ferrule Printing Machine ** No. 50000.00 Accessories & Spares 3 Black Ink Ribbon – {1 Box = 5 Rolls of 50 Mtrs} Box 3300.00 4 Ink Ribbon Case No. 850.00 5 Half Cutter Unit No. 1800.00 6 Adhesive Tape White / Yellow ( 8 mtr./Roll) 5 mm No. 650.00 7 Adhesive Tape White / Yellow ( 8 mtr./Roll) 9 mm No. 675.00 8 Adhesive Tape White / Yellow ( 8 mtr./Roll) 12 mm No. 750.00 9 Ferruling Tube, (100 m/Roll)-White [Suitable for 0.5 sq mm wire] 2.2 mm Roll 290.00 10 Ferruling Tube, (100 m/Roll)-White 2.5 mm Roll 290.00 11 Ferruling Tube, (100 m/Roll)-White [Suitable for 1 sq mm wire] 3.0 mm Roll 325.00 12 Ferruling Tube, (100 m/Roll)-White 3.2 mm Roll 340.00 13 Ferruling Tube, (100 m/Roll)-White [Suitable for 1.5 sq mm wire] 3.5 mm Roll 375.00 14 Ferruling Tube, (100 m/Roll)-White 4.0 mm Roll 385.00 15 Ferruling Tube, (100 m/Roll)-White [Suitable for 2.5 sq mm wire] 4.2 mm Roll 450.00 16 Ferruling Tube, (100 m/Roll)-White 4.7 mm Roll 470.00 17 Ferruling Tube, (100 m/Roll)-White [Suitable for 4 sq mm wire] 5.0 mm Roll 540.00 18 Ferruling Tube, (100 m/Roll)-White 5.5 mm Roll 575.00 19 Ferruling Tube, (100 m/Roll)-White [Suitable for 6 sq mm wire] 6.0 mm Roll 680.00 20 Ferruling Tube, (100 m/Roll)-White 6.3 mm Roll 775.00 21 Ferruling Tube, (100 m/Roll)-White [Suitable for 10 sq mm wire] 7.0 mm Roll 900.00 22 Ferruling Tube, (100 m/Roll)-White [Suitable for 16 sq mm wire] 8.0 mm Roll 1100.00 * Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Software CD , Manual , Carrying Case ** Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Manual Price Basis Ex-Godown, Kolkata.