Energy Flow and Food Web Ecology Along a Hydroperiod Gradient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecology of Aquatic Heteropters of Two Ponds
The Pharma Innovation Journal 2019; 8(7): 513-518 ISSN (E): 2277- 7695 ISSN (P): 2349-8242 NAAS Rating: 5.03 Ecology of aquatic heteropters of two ponds with TPI 2019; 8(7): 513-518 © 2019 TPI endemicity different from Buruli ulcer in the south of www.thepharmajournal.com Received: 28-05-2019 Cote d’ivoire (West Africa) Accepted: 30-06-2019 Bernard Kouadio Allali Bernard Kouadio Allali, Lambert K Konan, Nana R Diakite, Mireille Unité d'Entomologie et d'Herpétologie, Département Dosso and Eliezer KN Goran Environnement et Santé, Institut Pasteur de Côte d'Ivoire, Abstract 01 BP 490 Abidjan 01, Heteroptera generally colonize most aquatic ecosystems and play an important vector role in the spread Cote d'Ivoire of Buruli ulcer. This study aims to determine the diversity and distribution of Heteroptera species in the two pools of Sokrogbo and Vieil Aklodj in southern Côte d'Ivoire. Heteropteran fauna is sampled Lambert K Konan Unité d'Entomologie et monthly from January to December 2016 using a Troubleau net. Physico-chemical parameters of the d'Herpétologie, Département water bodies visited are determined using standard protocols. The results show a total of 35 taxa, Environnement et Santé, belonging to 9 families of heteropterans, at the two study sites. The number of taxa is respectively 32 and Institut Pasteur de Côte d'Ivoire, 25 at Sokrogbo and at Vieil Aklodj. Among the sampled taxa, Micronecta scutellaris, Diplonychus 01 BP 490 Abidjan 01, nepoïdes, Diplonychus sp., Anisops sp. and Ranatra fusca are the most abundant species. The indices Cote d'Ivoire Shannon Wiener, Equitability, Margalef and Evenness show that the Sokrogbo pond has a greater diversity than vieil Aklodj and that the differences are significant. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Article Full Text PDF (734KB)
RECORDS OF GUATEMALAN HEMIPTERA-HETEROPTERA WITH DESCRIPTION OF NEW SPECIES* HERBERT OSBORN and CARL J. DRAKE. The Guatemalan Hemiptera-Heteroptera listed and the new species described in this paper were collected by Prof. Jas. S. Hine during the winter of 1905. Altho most of the records re- corded herein are found in the "Biologia Centrali Americana" and confirm the records of Messrs. Distant and Champion, several are new to Guatemala and Honduras, some to Central America, and a few to science. Nearly all of the aquatic and semi-aquatic Heteroptera were turned over to Mr. J. R. de la Torre Bueno who has published a preliminary paper1 on the same. A paper2 covering part of the Homoptera was published by the senior author, but some of this material remains in the university collection for further study. Family CORIXID^E. Tenagobia socialis F. B. White. One specimen: Los Amates, Guatemala, Feb. 18th, 1905. Family NEPID^E. Ranatra fusca Palisot de Beauvois. Two typical specimens, taken at Los Amates, Guatemala, Jan. 16th, 1905. Family BELOSTOMID^E. Belostoma annulipes Herrich-Schaffer. One specimen: Los Amates, Guatemala, Jan. 16th, 1905. Abedus breviceps Stal. One specimen: Gualan, Guatemala, Jan. 14th, 1905. Zaitha anura Herrich-Schaffer. One specimen: Los Amates, Guatemala, Jan. 16th, 1905. Zaitha fusciventris Dufour. One specimen: Los Amates, Guatemala, Feb. 16th, 1905. Family GELASTOCORID^E. Pelogonus perbosci Guerin. Several specimens from Guatemala: Gualan, Jan. 14th; Los Amates, Feb. 16th; Santa Lucia, Feb. 2d, 1905. Gelastocoris oculatus Fabricius. Five specimens of this common and widely distributed species from Guatemala; Gualan, Jan. 14th; Aguas Callientes, Jan. -

SOP #: MDNR-WQMS-209 EFFECTIVE DATE: May 31, 2005
MISSOURI DEPARTMENT OF NATURAL RESOURCES AIR AND LAND PROTECTION DIVISION ENVIRONMENTAL SERVICES PROGRAM Standard Operating Procedures SOP #: MDNR-WQMS-209 EFFECTIVE DATE: May 31, 2005 SOP TITLE: Taxonomic Levels for Macroinvertebrate Identifications WRITTEN BY: Randy Sarver, WQMS, ESP APPROVED BY: Earl Pabst, Director, ESP SUMMARY OF REVISIONS: Changes to reflect new taxa and current taxonomy APPLICABILITY: Applies to Water Quality Monitoring Section personnel who perform community level surveys of aquatic macroinvertebrates in wadeable streams of Missouri . DISTRIBUTION: MoDNR Intranet ESP SOP Coordinator RECERTIFICATION RECORD: Date Reviewed Initials Page 1 of 30 MDNR-WQMS-209 Effective Date: 05/31/05 Page 2 of 30 1.0 GENERAL OVERVIEW 1.1 This Standard Operating Procedure (SOP) is designed to be used as a reference by biologists who analyze aquatic macroinvertebrate samples from Missouri. Its purpose is to establish consistent levels of taxonomic resolution among agency, academic and other biologists. The information in this SOP has been established by researching current taxonomic literature. It should assist an experienced aquatic biologist to identify organisms from aquatic surveys to a consistent and reliable level. The criteria used to set the level of taxonomy beyond the genus level are the systematic treatment of the genus by a professional taxonomist and the availability of a published key. 1.2 The consistency in macroinvertebrate identification allowed by this document is important regardless of whether one person is conducting an aquatic survey over a period of time or multiple investigators wish to compare results. It is especially important to provide guidance on the level of taxonomic identification when calculating metrics that depend upon the number of taxa. -
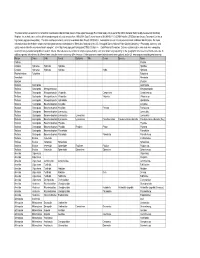
This Table Contains a Taxonomic List of Benthic Invertebrates Collected from Streams in the Upper Mississippi River Basin Study
This table contains a taxonomic list of benthic invertebrates collected from streams in the Upper Mississippi River Basin study unit as part of the USGS National Water Quality Assessemnt (NAWQA) Program. Invertebrates were collected from woody snags in selected streams from 1996-2004. Data Retreival occurred 26-JAN-06 11.10.25 AM from the USGS data warehouse (Taxonomic List Invert http://water.usgs.gov/nawqa/data). The data warehouse currently contains invertebrate data through 09/30/2002. Invertebrate taxa can include provisional and conditional identifications. For more information about invertebrate sample processing and taxonomic standards see, "Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory -- Processing, taxonomy, and quality control of benthic macroinvertebrate samples", at << http://nwql.usgs.gov/Public/pubs/OFR00-212.html >>. Data Retrieval Precaution: Extreme caution must be exercised when comparing taxonomic lists generated using different search criteria. This is because the number of samples represented by each taxa list will vary depending on the geographic criteria selected for the retrievals. In addition, species lists retrieved at different times using the same criteria may differ because: (1) the taxonomic nomenclature (names) were updated, and/or (2) new samples containing new taxa may Phylum Class Order Family Subfamily Tribe Genus Species Taxon Porifera Porifera Cnidaria Hydrozoa Hydroida Hydridae Hydridae Cnidaria Hydrozoa Hydroida Hydridae Hydra Hydra sp. Platyhelminthes Turbellaria Turbellaria Nematoda Nematoda Bryozoa Bryozoa Mollusca Gastropoda Gastropoda Mollusca Gastropoda Mesogastropoda Mesogastropoda Mollusca Gastropoda Mesogastropoda Viviparidae Campeloma Campeloma sp. Mollusca Gastropoda Mesogastropoda Viviparidae Viviparus Viviparus sp. Mollusca Gastropoda Mesogastropoda Hydrobiidae Hydrobiidae Mollusca Gastropoda Basommatophora Ancylidae Ancylidae Mollusca Gastropoda Basommatophora Ancylidae Ferrissia Ferrissia sp. -

Great Lakes Entomologist the Grea T Lakes E N Omo L O G Is T Published by the Michigan Entomological Society Vol
The Great Lakes Entomologist THE GREA Published by the Michigan Entomological Society Vol. 45, Nos. 3 & 4 Fall/Winter 2012 Volume 45 Nos. 3 & 4 ISSN 0090-0222 T LAKES Table of Contents THE Scholar, Teacher, and Mentor: A Tribute to Dr. J. E. McPherson ..............................................i E N GREAT LAKES Dr. J. E. McPherson, Educator and Researcher Extraordinaire: Biographical Sketch and T List of Publications OMO Thomas J. Henry ..................................................................................................111 J.E. McPherson – A Career of Exemplary Service and Contributions to the Entomological ENTOMOLOGIST Society of America L O George G. Kennedy .............................................................................................124 G Mcphersonarcys, a New Genus for Pentatoma aequalis Say (Heteroptera: Pentatomidae) IS Donald B. Thomas ................................................................................................127 T The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Missouri Robert W. Sites, Kristin B. Simpson, and Diane L. Wood ............................................134 Tymbal Morphology and Co-occurrence of Spartina Sap-feeding Insects (Hemiptera: Auchenorrhyncha) Stephen W. Wilson ...............................................................................................164 Pentatomoidea (Hemiptera: Pentatomidae, Scutelleridae) Associated with the Dioecious Shrub Florida Rosemary, Ceratiola ericoides (Ericaceae) A. G. Wheeler, Jr. .................................................................................................183 -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Paul P. Tinerella
University of Illinois Institute of Natural Resource Sustainability William Shilts, Executive Director ILLINOIS NATURAL HISTORY SURVEY Brian D. Anderson, Director 1816 South Oak Street Champaign, IL 61820 217-333-6830 INVENTORY OF AQUATIC TRUE BUGS (INSECTA: HETEROPTERA: NEPOMORPHA, GERROMORPHA, LEPTOPODOMORPHA) OF THE GREAT SMOKY MOUNTAINS NATIONAL PARK, NORTH CAROLINA AND TENNESSEE, USA Paul P. Tinerella Prepared for: DISCOVER LIFE IN AMERICA, INC. Grant / Project Number: DLIA2008-15 INHS Technical Report 2009 (22) Date of issue: 14 August 2009 COVER PAGE FOR FINAL REPORT TO DISCOVER LIFE IN AMERICA, INC. (Submit electronically with text of Final Report to [email protected]) PROPOSAL # DLIA2008-15 STARTING date: 1 April 2008 ENDING date: 1 March 2009 PRINCIPAL INVESTIGATOR (PI): Dr. Paul P. Tinerella PI DEPARTMENT: Illinois Natural History Survey PI ORGANIZATION: University of Illinois POSTAL ADDRESS: 1816 S Oak Street Champaign, IL 61820 PI ELECTRONIC MAIL: [email protected] PI TELEPHONE: 217-244-2149 PI FAX: 217-333-4949 TITLE of Project: INVENTORY OF AQUATIC TRUE BUGS (INSECTA: HETEROPTERA: NEPOMORPHA, GERROMORPHA, LEPTOPODOMORPHA) OF THE GREAT SMOKY MOUNTAINS NATIONAL PARK, NORTH CAROLINA AND TENNESSEE, USA GRANT AMOUNT: $4946.00 SUMMARY of Activities and Results (200 words; Lay Language): Research was conducted to document water bug (Insecta: Heteroptera: Nepomorpha, Gerromorpha, Leptopodomorpha) diversity of the Great Smoky Mountains National Park (GSMNP). Prior to this research, no water bug survey existed for GSMNP, with 13 total species historically recorded from the Park. One collecting trip of seven days (2-8 August 2008) was conducted in GSMNP, wherein 42 localities (lentic and lotic habitats) were sampled throughout the Park. -

Aquatic Insects Diversity and Water Quality Assessment of a Tropical Freshwater Pond in Benin City, Nigeria
PRINT ISSN 1119-8362 Full-text Available Online at J. Appl. Sci. Environ. Manage. Electronic ISSN 1119-8362 https://www.ajol.info/index.php/jasem Vol. 24 (7) 1129-1136 July 2020 http://ww.bioline.org.br/ja Aquatic Insects Diversity and Water Quality Assessment of a Tropical Freshwater Pond in Benin City, Nigeria 1, 2 * KOMOLAFE, BO; 2 IMOOBE, TOT *1Department of Zoology, Faculty of Science, University of Lagos, Akoka, Yaba, Lagos, Nigeria 2Department of Animal and Environmental Biology, Faculty of Life Science, University of Benin, Ugbowo, Benin City, Nigeria *Corresponding Author Email: [email protected] ABSTRACT: Aquatic insects are species of significant importance to water bodies because they serve various purposes including nutrient cycling, vectors of pathogens and bioindicators of water quality. Analyzing their community structure is a veritable tool in studies of biodiversity and quality of limnetic ecosystems. Therefore, we investigated the health status of a pond in Benin City, Nigeria using insect’s abundance, composition, distribution and physicochemical parameters of the waterbody. Insects were sampled using sweep nets and identified to the species level while water samples were collected and analyzed using in-situ and ex-situ methods to determine the physicochemical properties in three sampling stations. The results of the physicochemical assessment of the water indicated that conditions did not differ widely between sites (P > 0.05) except for total alkalinity, and the recorded values were well within the ambient FMEnv permissible limits for surface water except for dissolved oxygen, turbidity and phosphate. A total of 10 insect taxa, comprising of 103 individuals in 2 orders were recorded in the study and among the orders, Hemiptera comprised of 7 species and Coleoptera comprised of 3 species. -

(Hemiptera) for Arkansas, USA, As of 2018
Journal of the Arkansas Academy of Science Volume 71 Article 42 2017 Literature record checklist of true bugs (Hemiptera) for Arkansas, U.S.A., as of 2018. Stephen W. Chordas III Ohio State University, [email protected] Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Biodiversity Commons, Biology Commons, and the Entomology Commons Recommended Citation Chordas, Stephen W. III (2017) "Literature record checklist of true bugs (Hemiptera) for Arkansas, U.S.A., as of 2018.," Journal of the Arkansas Academy of Science: Vol. 71 , Article 42. Available at: http://scholarworks.uark.edu/jaas/vol71/iss1/42 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This General Note is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 71 [2017], Art. 42 Literature record checklist of true bugs (Hemiptera) for Arkansas, U.S.A., as of 2018 S.W. Chordas III Center for Life Sciences Education, Ohio State University, 260 Jennings Hall, 1735 Neil Avenue, Columbus, Ohio -
The Semiaquatic and Aquatic Hemiptera of California (Heteroptera: Hemiptera)
BULLETIN OF THE CALIFORNIA INSECT SURVEY Volume b21 The Semiaquatic and Aquatic Hemiptera of California (Heteroptera: Hemiptera) Edited by ARNOLD S. MENKE With contributions by Harold C. Chapman, David R. Lauck, Arnold S. Menke, John T: Polhemus, and Fred S. Truxal. THE SEMIAQUATIC AND AQUATIC HEMIPTERA OF CALIFORNIA (Hete ropt era: Hemiptera) BULLETIN OF THE CALIFORNIA INSECT SURVEY VOLUME 21 THE SEMIAQUATIC AND AQUATIC HEMIPTERA OF CALIFORNIA (Heteroptera: Hemiptera) c edited by ARNOLD S. MENKE with contributions by Harold C. Chapman, David R. Lauck, Arnold S. Menke, John T. Polhemus, and Fred S. Truxal UNIVERSITY OF CALIFORNIA PRESS BERKELEY*LOSANGELESLONDON BULLETIN OF THE CALIFORNIA INSECTSURVEY Advisory Editors: H. V. Daly. J. A. Powell, J. N,Belkin, R. M. Bohart, D. P. Furman, J. D. Pinto, E. I. Schlinger, R. W. Thorp VOLUME 21 UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES UNIVERSITY OF CALIFORNIA PRESS. LTD. LONDON. ENGLAND ISBN 0-520-09592-8 LIBRARY OF CONGRESS CATALOG CARD NUMBER 77-91755 0 1979 BY THE REGENTS OF THE UNIVERSITY OF CALIFORNIA PRINTED BY OFFSET IN THE UNITED STATES OF AMERICA ROBERT L. UStNGER initiated this project but did not live to see it ,finished. Bob was an inspirational teacher and n dear friend to all of us, and it is with great affection that we dedicate this bulletin to his memory. Contents Preface, ix Acknowledgments, x Abbreviations, xi INTRODUCTION, A. S. Menke California Fauna, 1 Habitat and Ecology, 2 Key to California semiaquatic and aquatic Hemiptera based on habitats and habits, 3 -

Una-Theses-0336.Pdf (2.298Mb Application/Pdf)
RKPORT or Committee on Thesis The undersigned, acting as a Committee of the Graduat~ School, have read the accompanying thesis subm'ltted. b y ···--··········:-:····-·'········eT··'" ···• ...'l. ....... ..... .. ................. l L ' -I ' ' · for the degree of ................ ............... ~.. ......... ........-- -·'···· ~ ... ................ They approve it as a thesis meeting the require- men.ts of the Gradu te School of t.he University of Minnesota, and recommend that it be accepted in partial fulfillment or the requirements for the degree of Chairman -···-·-····-·····-······························-·-··-······· ....... ~:... ... ··········· .......... .. .. -···-oo_• d~~ ..........-. .- ... .. ......·-·· - ············.. ····-- ······-··.. ···-·············-·A I II) .../!1.. , ..2.f l91 c. '' ' ,c ,' 1 tc 1 {r-. ~ ~ ' ' '"' A COHTRIBUTION TO THE LIFE HISTORY OF THE WATER SCORPION (Ranatra fusca, Pal.B.) ·• A Thesis submitted to the Faculty of the Graduate School of the University of Minnesota by Jean Plant ~ In partial fulfillment of the requirements for the degree of Master of Arts June 1916 0 u t 1 i n e . Introduction A. Behavior 1. General Responses under Normal Conditions 2. Special Reactions (1) Death-feigning (2) Reaction to Light B. Structural Modifications Summary and Conclusions 1. Contr ibution to the Life History of the Water Scorpion (Ranatra fusca, Pal.B.) Introduction. Ranatra fusca, the water scorpion, which is one of the con .nonest and best known Hemipteroi$J, affords an excellent opportun ity for a.n ecological study. The aim of this wor:t is to give the results of li1ve stigations made upon the adult from the standpoint of behavior, rather than to give any detailed account of the vari ous stages in the life history. In its best sense ecology in - eludes not only the study of an organism in its natural environ ment- that is the relation of organism to environment- but also the relation of envi ronment to the organism- briefly speaki ng the inter-action of organism and environment .