MS Analysis of Androgen Metabolites in Serum and Urine from East
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
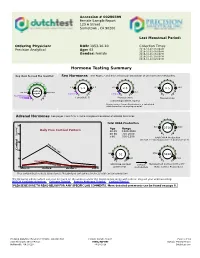
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

Testosterone, Or
COSI SIMILI, COSI DIVERSE 1. FOR A FEW ATOMS MORE: TESTOSTERONE AND DOPING A few days ago, the Tour de France winner, Floyd Landis, was found to have a high, indeed impermissible level of testosterone in his urine. Not quite, more of what was actually found in just a while. The sample was taken just after his comeback victory in a critical stage of bicycling’s premier race. If a second sample confirms the problem, Landis’s victory will be disallowed. Testosterone is the principal male sex hormone, produced mainly where… you might suspect from its name. And it is also produced in the ovaries of females. Testosterone is a so-called anabolic steroid, a class of molecules that give us a continuing lesson that almost the same is not the same. All the steroids, the class of molecules that include testosterone, have the same atomic framework – four all-carbon rings, fused together. Three are hexagons, the third ring going off at an angle to the other two. Fused to that last ring is a pentagon of carbon atoms. Call the rings A (6 carbons), B (6), C (6), D (5). Testosterone has an oxygen and a hydrogen (OH) attached to ring D, two CH3 (methyl) groups, one at the juncture of rings C and D, the other at the juncture of A and B. Finally ring A of testosterone has an oxygen attached to it as well, and there is a double bond in that ring. testosterone Testosterone is responsible for the secondary sex changes which occur in male puberty – facial and pubic hair, oiliness of skin, body odor, all that teenage boy stuff. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Steroid Profiling in Urine of Intact Glucuronidated and Sulfated
CORE Metadata, citation and similar papers at core.ac.uk Provided by Ghent University Academic Bibliography Journal of Chromatography A 1624 (2020) 461231 Contents lists available at ScienceDirect Journal of Chromatography A journal homepage: www.elsevier.com/locate/chroma Steroid profiling in urine of intact glucuronidated and sulfated steroids using liquid chromatography-mass spectrometry ∗ Laurie De Wilde , Kris Roels, Pieter Van Renterghem, Peter Van Eenoo, Koen Deventer 1 Doping Control Laboratory (DoCoLab), Ghent University (UGent), Department Diagnostic Sciences, Technologiepark 30B, B-9052 Zwijnaarde, Belgium a r t i c l e i n f o a b s t r a c t Article history: Detection of endogenous anabolic androgenic steroids (EAAS) misuse is a major challenge in doping con- Received 26 November 2019 trol analysis. Currently, a number of endogenous steroids, which constitute the steroid profile, are quanti- Revised 6 May 2020 fied using gas chromatography (GC). With this methodology, only the sum of the free and glucuronidated Accepted 10 May 2020 steroids is measured together. A dilute-and-shoot LC-MS method, which is compliant with the quality Available online 23 May 2020 requirements for measuring EAAS established by the World Anti-Doping Agency (WADA), was devel- Keywords: oped and validated containing glucuronidated and sulfated steroids in order to gain some extra infor- Doping mation and to expand the existing steroid profile. The developed method is, to the best of our knowl- Urine edge, the first method to combine both steroid glucuronides and sulfates, which is compliant with the Steroid profile quality standards of the technical document on EAAS, established by WADA. -

Criteria to Indicate Testosterone Administration
Br. J. Sp. Med; Vol 24, No. 4 Br J Sports Med: first published as 10.1136/bjsm.24.4.253 on 1 December 1990. Downloaded from Criteria to indicate testosterone administration A.T. Kicman1, R.V. Brooks2, S.C. Collyere, D.A. Cowan', M.N. Nanjee2, G.J. Southan2 and M.J. Wheelee 'Drug Control and Teaching Centre, King's College, London University 2Department of Chemical Pathology, UMDS, London University A detection method for testosterone administration was was found to increase after an intramuscular injection developed using radioimmunoassay to measure the of testosterone heptanoate in all ten normal males, urinary ratios of testosterone (T) to epitestosterone (E) and although there was an overlap between the basal to luteinizing hormone (LH). A comparative study of the range and that obtained three days after injection. effect on these ratios of a single intramuscular injection of testosterone heptanoate followed by stimulation with The method used was radioimmunoassay as protein human chorionic gonadotrophin (HCG) in three normal hormones cannot be measured by gas liquid chroma- men was undertaken. To allow immediate investigation, a tography-mass spectrometry (GLC-MS). commercially supplied epitestosterone antiserum was In 1983, the International Olympic Committee used. This study showed that both T/E and T/LH ratios (IOC) adopted the ratio of urinary testosterone to could be used to detect testosterone administration, the epitestosterone (T/E) as the sole test for testosterone latter also being an indicator of HCG use due to doping as both these hormones could be convenient- cross-reactivity with the LH antiserum. Subsequently, an ly measured by GLC-MS. -

Test Report Comprehensive Hormone Insights™
698814 COMPREHENSIVE HORMONE INSIGHTS™ TEST REPORT Dr. Maximus, N.D. E: [email protected] Date of Collection: P: 403-241-4500 Time of Collection: F: 403-241-4501 Date of Receipt: www.rmalab.com Reported On: CHI Accession: 698814 Healthcare Professional Patient Age: Dr. Maximus, N.D. Date of Birth: Gender: Male F: Relevant Medications Biometrics Curcumin Height (in) : 73 Weight (lb) : 180 BMI : 24 Waist (in) : 35 Hip (in) : 41 CHI Accession: 698814 SUMMARY HMUS01 How to read the graphs LEGEND: 50 66 Sex Steroid Hormones 50 66 Middle third of 33 33 84 reference population Hormone Start of 83 100 80 100 highest 16 Percentile Precursors 16 Percentile third of Sum of Androgens Sum of Estrogens 50 66 reference 0 0 population (T, DHT, α+β androstanediol) Listed in Interp Guide 33 84 End of 100 16 lowest Percentile00 50 66 50 66 third of 33 33 84 reference 0 population Patient’s percentile rank 81 100 95 100 compared to reference 16 Percentile 16 Percentile population (see summary) DHEA + Metabolites Sum of Progesterone Metabolites 0 (DHEA + A + E) 0 α+β Pregnanediol Cortisol Melatonin Oxidative Stress Free Cortisol Profile (ng/mg) 100 50 66 50 66 33 84 33 84 80 64 100 0 100 16 Percentile 16 Percentile 60 6-sulfatoxy 8-Hydroxy-2- 0 Melatonin 0 deoxyguanosine 40 (Overnight) (Overnight) 20 6-sulfatoxymelatonin provides 8-hydroxy-2-deoxyguanosine is Cortisol/Creatinine (ng/mg) insight into melatonin levels. a marker of oxidative stress 0 Morning Dinner Bedtime A B C 50 66 Free cortisol Cortisol Metabolites 33 84 profile is used to provides a general Testosterone Cortisol assess diurnal assessment of 16 100 cortisol rhythm adrenal cortisol 16 Percentile Cortisol production Cortisol Metabolites 0 (α+β THF + THE) Testosterone Cortisol/Testosterone provides insight into relative catabolic (cortisol) and anabolic (testosterone) states. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

169 2016 Interim Meeting Science and Public Health - 1
169 2016 Interim Meeting Science and Public Health - 1 REPORTS OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH The following reports, 1–4, were presented by S. Bobby Mukkamala, MD, Chair: 1. URINE DRUG TESTING Reference committee hearing: see report of Reference Committee K. HOUSE ACTION: RECOMMENDATIONS ADOPTED AS FOLLOWS REMAINDER OF REPORT FILED See Policies H-95.985 and D-120.936 INTRODUCTION Over the past two decades, the rate of opioid prescribing, especially for patients with chronic non-cancer pain, has increased dramatically. It is estimated that between 9.6 and 11.5 million Americans are currently being prescribed long-term opioid therapy.1 The overall increase in prescribing has been associated with a parallel increase in unintentional overdoses and deaths from prescription opioids.2 In 2014, a total of 47,055 drug overdose deaths occurred in the United States; 61% of these involved some type of opioid, including heroin. Overdose deaths from heroin have quadrupled in recent years, and the majority of past year users of heroin report they used opioids in a nonmedical fashion prior to heroin initiation; hence, the availability of pharmaceutical opioids is relevant to the national heroin use and overdose death epidemics. In the most recent available report, benzodiazepines were involved in 31% of the opioid-related overdoses.3 Despite clinical recommendations to the contrary, the rate of opioid and benzodiazepine co-prescribing also continues to rise.3-5 Identifying patients at risk for drug misuse is a challenge. There is no definitive way for physicians to predict which of their patients will develop misuse problems with controlled substances. -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

Normal Steroid Levels in Racehorses (Print) Page 1 of 2
The Horse | Normal Steroid Levels in Racehorses (print) Page 1 of 2 Normal Steroid Levels in Racehorses by: Christy West February 28 2010 Article # 15889 Steroid usage in racehorses has received a good deal of attention in the media, perhaps reaching a peak during the 2009 Triple Crown season when Big Brown won the Kentucky Derby and Preakness on the legally administered steroid stanozolol, then flopped in the Belmont without it. While no one could ever prove the steroid helped the horse win or that his loss was associated with being steroid-free, the situation added significant fuel to the fire of medication regulation in racehorses. One of the tough aspects of regulating substances that are naturally produced in the horse's body, such as many steroids, is that before you can decide how high a level of the substance constitutes an administered medication or abuse, you have to find out how much horses produce normally. At the 2009 American Association of Equine Practitioners (AAEP) Convention held Dec. 5-9 in Las Vegas, Nev., one presenter discussed a study that sought to answer that question for anabolic androgenic steroids (hormones that stimulate masculine physical characteristics) in young Thoroughbreds. Currently there are four anabolic androgenic steroids commonly used therapeutically in racehorses: Stanozolol, nandrolone, testosterone, and boldenone, said presenter Benjamin C. Moeller, BS, a graduate student at the K.L. Maddy Equine Analytical Chemistry Laboratory at the University of California, Davis. In collaboration with veterinarians at Rood & Riddle Equine Hospital and Hagyard Equine Medical Institute (both in Lexington, Ky.), and Craig Van Balen (private practitioner in Lexington), 142 un-medicated Thoroughbred colts and 62 fillies in training at Central Kentucky farms, from five to 24 months of age, were blood tested monthly for 13 months. -

Pharmaceutical and Veterinary Compounds and Metabolites
PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES High quality reference materials for analytical testing of pharmaceutical and veterinary compounds and metabolites. lgcstandards.com/drehrenstorfer [email protected] LGC Quality | ISO 17034 | ISO/IEC 17025 | ISO 9001 PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES What you need to know Pharmaceutical and veterinary medicines are essential for To facilitate the fair trade of food, and to ensure a consistent human and animal welfare, but their use can leave residues and evidence-based approach to consumer protection across in both the food chain and the environment. In a 2019 survey the globe, the Codex Alimentarius Commission (“Codex”) was of EU member states, the European Food Safety Authority established in 1963. Codex is a joint agency of the FAO (Food (EFSA) found that the number one food safety concern was and Agriculture Office of the United Nations) and the WHO the misuse of antibiotics, hormones and steroids in farm (World Health Organisation). It is responsible for producing animals. This is, in part, related to the issue of growing antibiotic and maintaining the Codex Alimentarius: a compendium of resistance in humans as a result of their potential overuse in standards, guidelines and codes of practice relating to food animals. This level of concern and increasing awareness of safety. The legal framework for the authorisation, distribution the risks associated with veterinary residues entering the food and control of Veterinary Medicinal Products (VMPs) varies chain has led to many regulatory bodies increasing surveillance from country to country, but certain common principles activities for pharmaceutical and veterinary residues in food and apply which are described in the Codex guidelines. -

Genetics of Androgen Disposition
From the Department of Laboratory Medicine Division of Clinical Pharmacology Karolinska Institutet, Stockholm, Sweden GENETICS OF ANDROGEN DISPOSITION - Implications for Doping Tests Jenny Jakobsson Schulze Stockholm 2007 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. © Jenny Jakobsson Schulze, 2007 ISBN 978-91-7357-397-9 Printed by 2007 Gårdsvägen 4, 169 70 Solna One must sit down before truth without preconception, like a little child, and follow where the facts lead- or one will learn nothing. Thomas Huxley (1825-1895) To Joe and Dante ABSTRACT Anabolic androgenic steroids (AAS) are derivatives of testosterone. Doping with AAS is a severe challenge to the vision, moral and ethics in sports and has also become an increasing problem in society. Testosterone abuse is conventionally assessed by the urinary testosterone glucuronide/ epitestosterone glucuronide (T/E) ratio, levels above 4.0 being considered suspicious. However, there is a large inter-individual variation in testosterone glucuronide and epitestosterone glucuronide excretion, which challenges the accuracy of the test. There are reasons to believe that genetic variation is the single most important cause of variation in disposition of many androgenic compounds. Twin studies in men have demonstrated heritability estimates of 85% and 96% for production rates of testosterone and dihydrotestosterone, respectively. The primary aim of this thesis was to investigate the contribution of genetic components to inter-individual variation in androgen disposition. We found that a deletion polymorphism in the UGT2B17 gene was strongly associated with the urinary testosterone glucuronide levels. All individuals homozygous for the deletion had negligible amounts of urinary testosterone glucuronide.