RESTAURANT FOOD WASTE MANAGEMENT USING MICROWAVE PLASMA GASIFICATION TECHNOLOGY VIJAYAKUMAR KARUNAMOOTHEI a Thesis Submitted In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bovine Benefactories: an Examination of the Role of Religion in Cow Sanctuaries Across the United States
BOVINE BENEFACTORIES: AN EXAMINATION OF THE ROLE OF RELIGION IN COW SANCTUARIES ACROSS THE UNITED STATES _______________________________________________________________ A Dissertation Submitted to the Temple University Graduate Board _______________________________________________________________ In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY ________________________________________________________________ by Thomas Hellmuth Berendt August, 2018 Examing Committee Members: Sydney White, Advisory Chair, TU Department of Religion Terry Rey, TU Department of Religion Laura Levitt, TU Department of Religion Tom Waidzunas, External Member, TU Deparment of Sociology ABSTRACT This study examines the growing phenomenon to protect the bovine in the United States and will question to what extent religion plays a role in the formation of bovine sanctuaries. My research has unearthed that there are approximately 454 animal sanctuaries in the United States, of which 146 are dedicated to farm animals. However, of this 166 only 4 are dedicated to pigs, while 17 are specifically dedicated to the bovine. Furthermore, another 50, though not specifically dedicated to cows, do use the cow as the main symbol for their logo. Therefore the bovine is seemingly more represented and protected than any other farm animal in sanctuaries across the United States. The question is why the bovine, and how much has religion played a role in elevating this particular animal above all others. Furthermore, what constitutes a sanctuary? Does -

FOI Ref 6871 Response Sent 17 March Can You Please Provide The
FOI Ref 6871 Response sent 17 March Can you please provide the following details from the most recent records which you hold under The Licensing Act 2003: On-trade alcohol licensed premises, including: Premises Licence Number Date Issued Premises Licence Status (active, expired etc.) Premises Name Premises Address Premises Postcode Premises Telephone Number Premises on-trade Category (e.g. Cafe, Bar, Theatre, Nightclub etc.) Premises Licence Holder Name Premises Licence Holder Address Premises Licence Holder Postcode Designated Premises Supervisor Name Designated Premises Supervisor Address Designated Premises Supervisor Postcode The information you have requested is held and in the attached. However the personal information relating to Designated Premises Supervisors you have requested is refused under the exemption to disclosure at Section 40(2) of the Act Further queries on this matter should be directed to [email protected] Full address Telephone number Licence type Status Date issued Licence holder 1 and 1 Rougamo Ltd, 84 Regent Street, Cambridge, 07730029914 Premises Licence Current Licence 10/03/2020 Yao Qin Cambridgeshire, CB2 1DP 2648 Cambridge, 14A Trinity Street, Cambridge, 01223 506090 Premises Licence Current Registration 03/10/2005 The New Vaults Limited Cambridgeshire, CB2 1TB 2nd View Cafe - Waterstones, 20-22 Sidney Street, Premises Licence Current Registration 17/09/2010 Waterstones Booksellers Ltd Cambridge, Cambridgeshire, CB2 3HG ADC Theatre, Park Street, Cambridge, Cambridgeshire, CB5 01223 359547 Premises Licence -

How to Avoid Making BIG Mistakes with Your Nutrition When Eating out Or Ordering Takeaway Aivaras &
THE ULTIMATE EATING OUT CHEAT SHEET! How to avoid making BIG mistakes with your nutrition when eating out or ordering takeaway Aivaras & Joe Fitness Experts One of the biggest nutritional questions I get asked is: Does one night out really matter? Much to my clients’ dismay my answer is always YES! When it comes to nutrition, consistency is key. Why ruin a week where you have stuck hard to your diet plan, visited the gym 3-4 times, and said no to all those tempting treats, by consuming a calorie-laden meal that will cause your blood sugar to spike and will often lead to other temptations?! So what do you do? Forgo any fun and never go out again in order to stick to your nutritionally healthy lifestyle? No! That is simply unsustainable… the answer is to eat smart. One night out does matter if you are going to throw caution to the wind and eat whatever you feel like. BUT if you’re smart - and think about the nutritional content of the meal you’re ordering and make healthy swaps - eating out can be as healthy as eating in! The key is knowing the nutritional content of what’s on your plate and to help you do this I have produced this helpful eBook packed full of great information about what to choose and what to lose. The ‘Ultimate Eating Out Cheat Sheet’ has been specifically designed to help you make the right choices and make eating out less daunting. So remember, healthy eating is not about depriving yourself; it’s about eating smarter! DISCLAIMER All food items we have presented in this document were found during online searches; as a result some are missing specific components due to the companies involved not posting all the information. -

Casual Dining Is the Best Show out There for Pubs and Restaurants That Want to Find Innovative New Products and Inspiration.”
Connecting you with the biggest buyers in the sector “Casual Dining is the best show out there for pubs and restaurants that want to find innovative new products and inspiration.” ROSS PIKE, CHEF DIRECTOR, OAKMAN INNS RESTAURANTS | PUBS | BARS | WHOLESALERS | DISTRIBUTORS | HOTELS | CONTRACT CATERERS Welcome to Casual DINING THE UK CASUAL DINING SECTOR IS A MULTI BILLION POUND INDUSTRY. BE A PART OF IT. Casual Dining is the two days a year when the sector gets together for innovative product launches, FREE world class seminars, networking and to do business. With over 220 suppliers and more than 5000 of the biggest buyers, Casual Dining is still the only trade show completely dedicated, targeted and focused on the fast-moving casual dining sector. Casual dining operators are capitalising on emerging consumer trends to help them flourish in this current environment. Exciting innovations from new and existing brands are increasingly important which makes the Casual Dining show more relevant than ever. Who exhibits? Exhibiting companies at Casual Dining showcase a variety of innovative products and services including: FOOD | CATERING EQUIPMENT TEA | COFFEE | HOT DRINKS | SOFT DRINKS | JUICES | MIXERS BEER | CIDER | WINE | SPIRITS TABLETOP | BAR EQUIPMENT | FRONT OF HOUSE TECHNOLOGY | DELIVERY SERVICES | EPOS DESIGN SERVICES | FURNITURE | RECRUITMENT | FINANCIAL SERVICES “Casual Dining is unquestionably our most important trade show in the UK as it is the most directly focused show in our sector.” IAN R. RONAN, SENIOR BUSINESS DEVELOPMENT MANAGER, SEA PRODUCTS INTERNATIONAL LTD Do you want to do business with... RESTAURANTS, PUBS, BARS, HOTELS and more? Exhibitors at the 2019 show met key decision makers from.. -

Sale of Byron to Hutton Collins Partners
Friday 18th October 2013 Sale of Byron to Hutton Collins Partners The Gondola Group and Hutton Collins today announce that they have reached agreement for funds managed/advised by Hutton Collins Partners LLP to acquire Byron Hamburgers Limited (“Byron”), the UK burger restaurant chain, for a consideration of £100 million. Byron was founded by The Gondola Group in 2007 with a mission to deliver the perfect burger to the UK market. It has been highly innovative since conception with a proposition based around a simple menu of classic hamburgers. Its restaurants, which are individually tailored to each particular property, locality and customer audience, retain Byron’s strong brand identity. In six years, Byron has rapidly grown to 34 sites and has been successfully transformed from an emerging concept to a proven business with exciting prospects for further growth and a well-advanced pipeline of openings. Primarily focused on London, Byron has now expanded beyond the capital with strong openings being achieved in a number of key regional cities. Byron’s highly experienced management team, led by Tom Byng, will remain with the business. Harvey Smyth, Chief Executive of The Gondola Group, commented: ‘Byron has gone from strength to strength since we launched it in 2007. Its passion and innovative approach have already positioned it as an important brand in the casual dining market, with exciting opportunities to continue to grow throughout the UK. The team at Byron have done a fantastic job and they leave The Gondola Group with our thanks and best wishes for the future.’ Graham Hutton, Managing Partner at Hutton Collins, added: ‘We are delighted to be investing in such a pioneering brand. -

MCR Student Social
MANCHESTER’S ULTIMATE SHOPPING NIGHT SHOP. SAVE. PLAY! An exclusive night of big shopping discounts, music and entertainment just for you MANCHESTER ARNDALE, MARKET ST, ST ANN’S SQUARE, NEW CATHEDRAL ST, EXCHANGE SQUARE, KING ST, DEANSGATE # BlackMARKET Dog Ballroom's STREET live DJs play all the hits ST ANN'S SQUARE Macarena, Y.M.C.A., Gangnam Style… — dance the day away and win prizes Big Bad Burger Pong! Beat your mates and with Capital FM's silent disco win big with Byron Hamburgers. Plus 50% off at Byron Hamburgers all night long — Test your boxing skills with Pure Gym's expert personal trainers EXCHANGE SQUARE — Kiehl's on Wheels pop-up beauty Spin to win with Barburrito store; skin consultations, offers & — free samples Win a holiday to Ibiza with Black Dog — Ballroom, plus pick up your goody bag Win offers and prizes at and your exclusive Student Dog Tag for The Printworks' life-sized Hungry discounts all year Hippo game — Take on the Breakout Manchester challenge to test your skill, logic and brain power, will you beat them? They think not! MANCHESTER ARNDALE — EXCHANGE COURT 0UTSIDE NEXT Matt & Phreds presents Heavy Beat Join Three for the chance to win Brass Band roaming gig a Samsung prize package worth — over £1,000 4-9pm Take part in the UNiDAYS challenge for — # your chance to win amazing prizes Everyone’s a winner with Greggs! — Come and play ‘Lucky Lights’ to see Enjoy footy themed fun and frolics on the which prize you could win ILoveMCR life-sized human foosball pitch — Grab a bite in the Foodcourt and enjoy live acoustic performances from Mike Ritchie & Laura Kelly MCRGet set FRESHER'Sfor the NEW TERM. -

FOI Request 8139
FOI Ref Response sent 8139 10 Nov 20 (CCC) Premise License Premise License Please could you provide a list of all premises granted a license to sell alcohol. Response: Thank you for your request for information above, which we have dealt with under the terms of the Freedom of Information Act 2000. I hope the following will answer your query: This information is already accessible online on our website at: https://licences.cambridge.gov.uk/Registers_Criteria.aspx; however, for your convenience I have attached a list of all businesses currently granted an active Premises Licence by Cambridge City Council to sell alcohol by retail. Further queries on this matter should be directed to [email protected] Address @72.China, 72 Regent Street, Cambridge, Cambridgeshire, CB2 1DP. 1 and 1 Rougamo Ltd, 84 Regent Street, Cambridge, Cambridgeshire, CB2 1DP. 196 Restaurant & Cocktail Bar, 196 Mill Road, Cambridge, Cambridgeshire, CB1 3NF. 2648 Cambridge, 14A Trinity Street, Cambridge, Cambridgeshire, CB2 1TB. 2nd View Cafe - Waterstones, 20-22 Sidney Street, Cambridge, Cambridgeshire, CB2 3HG. ADC Theatre, Park Street, Cambridge, Cambridgeshire, CB5 8AS. Agora at The Copper Kettle, 3-4 Kings Parade, Cambridge, Cambridgeshire, CB2 1SJ. Al Casbah Restaurant, 62 Mill Road, Cambridge, Cambridgeshire, CB1 2AS. Al Pomodoro, 8 Homerton Street, Cambridge, Cambridgeshire, CB2 8NX. Aldi Store, 393 Newmarket Road, Cambridge, Cambridgeshire, CB5 8JL. Aldi, Unit 1, 157 Histon Road, Cambridge, Cambridgeshire, CB4 3JD. All Bar One, All Bar One, 36 St Andrews Street, Cambridge, Cambridgeshire, CB2 3AR. Amelie Restaurants, Grafton Centre, Fitzroy Street, Cambridge, Cambridgeshire. Anglia Ruskin University, East Road, Cambridge, Cambridgeshire, CB1 1PT. -

Download Where Retail Happens 2016 Issue 3
150.5mm 10mm 150.5mm 2016 2016 Issue 3/Autumn 2016 happens retail Where Where retailWhere happens At Hammerson, we create destinations that excite shoppers, attract and support retailers, reward investors and serve communities; destinations where more happens. hammerson.com [email protected] 210mm London Kings Place, 90 York Way, London N1 9GE +44 (0) 20 7887 1000 Paris 48 rue Cambon 75001 Paris +33 (0)1 56 69 30 00 Dublin Harcourt Centre, Harcourt Street, Dublin 2 +353 86 248 9065 BACK COVER SPINE OUTER FACE / FRONT COVER 150.5mm 10mm 150.5mm Aberdeen Belfast Birmingham Bristol Didcot Dublin Falkirk Glasgow Gloucester Kirkcaldy Where fashion happens Leeds Where fun happens Leicester Where footfall happens London Where retail happens Luton Where retail happens We are Hammerson. As a leading Middlesbrough owner-manager and developer of retail Paisley destinations, we are proud to be behind Reading some of the greatest retail experiences 210mm Rugby in Europe. Our £10 billion real estate Southampton portfolio comprises 22 prime regional Swansea shopping centres, 19 convenient retail Thanet parks and investments in 15 premium designer outlet villages which draw Our portfolio and excite shoppers and attract and Aulnay-sous-Bois support retailers. Our success is the result of putting more time, more care, more Beauvais knowledge and more imagination into Cergy-Pontoise our projects. By doing this, we create Marseille destinations where more happens. Nancy Nice Paris Saint-Quentin-en-Yvelines Strasbourg SPINE DIRECTORY BOOK INSERT FIXED HERE WELCOMING OVER 2 8 0 MILLION CUSTOMERS TO Where 02 retail 03 – happens 02 OUR DESTINATIONS EACH Our Product Experience Framework is embedded across everything we do, YEAR providing a unique point of differentiation to our operational model. -

Mintel Reports Brochure
Burger and Chicken Restaurants - UK - August 2017 The above prices are correct at the time of publication, but are subject to Report Price: £1995.00 | $2583.33 | €2370.86 change due to currency fluctuations. “Parents are vital to burger and chicken restaurants as the population of 0-14 year-olds grows. Brands are focusing on non-core items to boost snacking occasions, while diners want to see less sugar, fat and salt. Yet the sector is experiencing the halo effect, with diners interested in gourmet chicken. Meanwhile, daily deals, loyalty cards and late-night openings can nudge more diners to visit.” – Trish Caddy, Foodservice Analyst This report looks at the following areas: BUY THIS • It’s vital to engage parents of under-16s REPORT NOW • There’s scope to cater to older diners • Non-core items are important in their own way VISIT: Much of the growth in the burger and chicken restaurants market is due to brands pushing to build store.mintel.com consumer trust, new entrants’ expansion plans, efforts to innovate in non-core products to boost snacking occasions and a focus on high-welfare ingredients and healthier cooking methods. This is despite UK restaurant groups facing headwinds to maintain trading levels as their margins are CALL: squeezed by increasing overheads. EMEA +44 (0) 20 7606 4533 The forecast population growth of 0-14 year-olds between 2016 and 2021 bodes well, given that parents of under-16s are most likely to visit a burger or chicken outlet/restaurant. Daily deals, loyalty cards and late-night openings can also nudge more diners to visit a burger or chicken restaurant. -

Mintel Reports Brochure
Burger and Chicken Restaurants - UK - September 2018 The above prices are correct at the time of publication, but are subject to Report Price: £1995.00 | $2693.85 | €2245.17 change due to currency fluctuations. “The biggest threat to the popularity of burger and chicken is the trend of consumers cutting back on eating meat. This is being driven by Younger Millennials who have either adopted a full-time vegan lifestyle or are simply eating more plant-based dishes. Operators now need to tackle this issue by offering consumers more varied choice, including vegan burgers.” – Trish Caddy, Foodservice Analyst This report looks at the following areas: BUY THIS • A meaty issue REPORT NOW • A sweet problem • Just how price-conscious are Millennials? VISIT: Much of the growth in the burger and chicken restaurants market has been driven by brands pushing store.mintel.com digital innovation and developing new takeaway and home delivery options. Meanwhile, sit-down restaurant chains have become destination businesses through offering healthier options, a higher quality of food, and a greater customer experience. CALL: EMEA The biggest threat to the popularity of burgers and chicken is the trend of consumers cutting back on +44 (0) 20 7606 4533 eating meat. Meanwhile, the frugal mentality continues to be deeply ingrained, which is likely to have some impact on discretionary spending on eating and drinking out-of-home. Brazil The forecast population growth of 5-14-year-olds over the next five years bodes well, given that 0800 095 9094 parents of under-16s are most likely to visit a burger or chicken outlet/restaurant on a regular basis. -
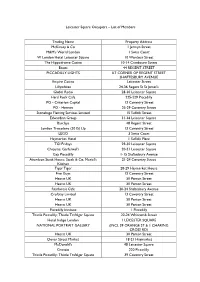
Leicester Square Occupiers – List of Members Trading Name Property Address Mckinsey & Co 1 Jermyn Street M&M's World
Leicester Square Occupiers – List of Members Trading Name Property Address McKinsey & Co 1 Jermyn Street M&M's World London 1 Swiss Court W London Hotel Leicester Square 10 Wardour Street The Hippodrome Casino 10-14 Cranbourn Street Boots 44 REGENT STREET PICCADILLY LIGHTS AT CORNER OF REGENT STREET SHAFTESBURY AVENUE Empire Casino Leicester Street Lillywhites 24-36 Regent St St James's Global Radio 28-30 Leicester Square Hard Rock Cafe 225-229 Piccadilly PO - Criterion Capital 13 Coventry Street PO - Hermes 25-29 Coventry Street Stonehage Fleming Services Limited 15 Suffolk Street Edwardian Group 31-36 Leicester Square Barclays 48 Regent Street London Trocadero (2015) Llp 13 Coventry Street LEGO 3 Swiss Court Haymarket Hotel 1 Suffolk Place TGI Fridays 29-30 Leicester Square Chiquito; Garfunkel's 20-21 Leicester Square Gap Piccadilly 1-15 Shaftesbury Avenue Aberdeen Steak House; Steak & Co; Muriel's 21-24 Coventry Street Kitchen Tiger Tiger 28-29 Haymarket House Five Guys 13 Coventry Street Hearst UK 30 Panton Street Hearst UK 30 Panton Street Rainforest Cafe 20-24 Shaftesbury Avenue Croftray Limited 13 Coventry Street Hearst UK 30 Panton Street Hearst UK 30 Panton Street Piccadilly Institute 1 Piccadilly Thistle Piccadilly; Thistle Trafalgar Square 22-24 Whitcomb Street Hotel Indigo London 1 LEICESTER SQUARE NATIONAL PORTRAIT GALLERY (INCL 39 ORANGE ST & 1 CHARING CROSS RD) Hearst UK 30 Panton Street Dover Street Market 18-21 Haymarket McDonald's 48 Leicester Square Granaio 220 Piccadilly Thistle Piccadilly; Thistle Trafalgar Square -

New Mersey Shopping Park Speke, Liverpool L24 8Qb
NEW MERSEY SHOPPING PARK SPEKE, LIVERPOOL L24 8QB SCHEME DETAILS 1 River Island 6,277 sq ft Y E L H S 2 Clintons 5,050 sq ft A A R U A 2A New Look 7,420 sq ft L 3 Sports Direct 15,000 sq ft Builders Yard 4A/B Marks & Spencer 20,147 sq ft FOOTASYLUM 5 Next 35,000 sq ft 7 JD Sports 10,000 sq ft 8A1 The Carphone Warehouse 4,000 sq ft 8A1B Costa Coffee 950 sq ft 8A2 WH Smith 4,950 sq ft UNIT L4 8B H&M 10,000 sq ft 9/10 Boots 24,149 sq ft 10A Clarks 5,000 sq ft 10C Currys / PC World 15,000 sq ft 11A Thomson Holiday 5,000 sq ft 11B Laura Ashley 5,000 sq ft Garden Centre A1 Sofology 10,258 sq ft A2 Halfords 8,112 sq ft TO LIVERPOOL A3 Argos 10,011 sq ft CITY CENTRE A4 ScS 10,278 sq ft PLANNING SECURED: A5 Marks & Spencer Simply Food 10,073 sq ft 11 SCREEN CINEWORLD CINEMA K1 DFS 21,193 sq ft OPENING SPRING 2017 K2A Pets at Home 10,070 sq ft A561 SPEKE ROAD Continued overleaf 06/16 TO M56 MOTORWAY OWNERSHIP SCHEME SIZE PLANNING CAR PARKING The Hercules Unit Trust 436,000 sq ft (40,506 sq m) Part Open A1/Part Restricted 1,850 (managed by British Land) Retail Leisure TOBY SYKES MICHAELA DAKIN THOMAS ROSE T: 020 7152 5240 T: 020 7152 5546 T: 020 7152 5278 E: [email protected] E: [email protected] E: [email protected] NEW MERSEY SHOPPING PARK SPEKE, LIVERPOOL L24 8QB SCHEME DETAILS K2B Harveys 9,830 sq ft Y E L H S L1A Foot Asylum 5,000 sq ft A A R U A L1B Mamas & Papas 5,000 sq ft L L2A Smyths Toys 13,017 sq ft Builders Yard LB2 Tessuti 4,786 sq ft FOOTASYLUM L3 Outfit 12,800 sq ft L4 TO LET 10,229 sq ft L5 Mothercare / PixiFoto