R10825cp (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
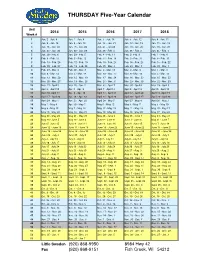
Thursday Calendar 20142018
THURSDAY Five-Year Calendar Unit 2014 20152016 2017 2018 Week # 1 Jan. 2 - Jan. 9 Jan. 1 - Jan. 8 Jan. 7 - Jan. 14 Jan. 5 - Jan. 12 Jan. 4 - Jan. 11 2 Jan. 9 - Jan. 16 Jan. 8 - Jan. 15 Jan. 14 - Jan. 21 Jan. 12 - Jan. 19 Jan. 11 - Jan. 18 3 Jan. 16 - Jan. 23 Jan. 15 - Jan. 22 Jan. 21 - Jan 28 Jan. 19 - Jan. 26 Jan. 18 - Jan. 25 4 Jan. 23 - Jan. 30 Jan. 22 - Jan. 29 Jan. 28 - Feb. 4 Jan. 26 - Feb. 2 Jan. 25 - Feb. 1 5 Jan. 30 - Feb. 6 Jan. 29 - Feb. 5 Feb. 4 - Feb. 11 Feb. 2- Feb. 9 Feb. 1 - Feb. 8 6 Feb. 6 - Feb. 13 Feb. 5 - Feb. 12 Feb. 11 - Feb. 18 Feb. 9 - Feb. 16 Feb. 8 - Feb. 15 7 Feb. 13 - Feb. 20 Feb. 12 - Feb. 19 Feb. 18 - Feb. 25 Feb. 16 - Feb. 23 Feb. 15 - Feb. 22 8 Feb. 20 - Feb. 27 Feb. 19 - Feb. 26 Feb. 25 - Mar. 3 Feb. 23 - Mar. 2 Feb. 22 - Mar. 1 9 Feb. 27 - Mar. 6 Feb. 26 - Mar. 5 Mar. 3 - Mar. 10 Mar. 2 - Mar. 9 Mar. 1 - Mar. 8 10 Mar. 6 - Mar. 13 Mar. 5 - Mar. 12 Mar. 10 - Mar. 17 Mar. 9 - Mar. 16 Mar. 5 - Mar. 15 11 Mar. 13 - Mar. 20 Mar. 12 - Mar. 19 Mar. 17 - Mar. 24 Mar. 16 - Mar. 23 Mar. 15 - Mar. 22 12 Mar. 20 - Mar. 27 Mar. 19 - Mar. 26 Mar. 24 - Mar. 31 Mar. 23 - Mar. 30 Mar. 22 - Mar. -

Laparoscopic Gastropexy As a Preventative Measure for Gastric Dilation Volvulus in Canines
Laparoscopic Gastropexy as a Preventative Measure for Gastric Dilation Volvulus in Canines By: Erin O’Brien Advisors: Dr. Kimberly Boswell Board Certified Surgeon Southwest Michigan Animal Emergency Hospital Kalamazoo, MI Dr. Diane R. Kiino Ph.D. Kalamazoo College Health Science A paper submitted in partial fulfillment of the requirements for the degree of Bachelor of Arts at Kalamazoo College. 2010 ii ACKNOWLEDGEMENTS Over the summer I was able to intern at the Southwest Michigan Animal Emergency Hospital in Kalamazoo, MI. It was there that I was exposed to the emergency setting in veterinary medicine but also had the chance to observe surgeries done by Board Certified Surgeon, Dr. Kimberly Boswell. I would like to thank the entire staff at SWMAEH for teaching me a tremendous amount about veterinary medicine and allowing me to get as much hands on experience as possible. It was such a privilege to complete my internship at a hospital where I was able to learn so much about veterinary medicine in only ten weeks. I would also like to thank Dr. Boswell in particular, it was a gastropexy surgery I saw her perform during my internship that inspired the topic of this paper. Additionally I would like to acknowledge my advisor Dr. Diane Kiino for providing the direction I needed in choosing my paper topic. iii ABSTRACT Gastric Dilation Volvulus (GDV) is a fatal condition in canines especially those that are large or giant breeds. GDV results from the stomach distending and twisting on itself which when left untreated causes shock and ultimately death. The only method of prevention for GDV is a gastropexy, a surgical procedure that sutures the stomach to the abdominal wall to prevent volvulus or twisting. -

Laparoscopic Fundoplication with Double Sided Posterior Gastropexy: a Different Surgical Technique
View metadata, citation and similar papers at core.ac.uk ORIGINAL RESEARCH brought to you by CORE provided by Elsevier - Publisher Connector International Journal of Surgery 10 (2012) 532e536 Contents lists available at SciVerse ScienceDirect International Journal of Surgery journal homepage: www.theijs.com Original research Laparoscopic fundoplication with double sided posterior gastropexy: A different surgical technique Fahri Yetis¸ira,*, A. Ebru Salman b,Dogukan Durak a, Mehmet Kiliç c a Ataturk Research and Training Hospital, General Surgery Department, Turkey b Ataturk Research and Training Hospital, Anesthesiology and Reanimation Department, Turkey c Yildirim Beyazit University, General Surgery Department, Turkey article info abstract Article history: Background: Laparoscopic Nissen Fundoplication has become the gold standard surgical procedure for Received 18 April 2012 management of gastroesophageal reflux disease. Nissen fundoplication provides an effective barrier Received in revised form against reflux. The aim of this study was to evaluate early postoperative outcomes of a different surgical 3 August 2012 technique, laparoscopic fundoplication with double sided posterior gastropexy. Accepted 6 August 2012 Methods: Data of 46 patients who underwent laparoscopic fundoplication with double sided posterior Available online 21 August 2012 gastropexy between February 2010 and December 2011 were collected. Surgically, after Nissen fundoplication was completed, 2e4 sutures were passed through the uppermost parts of the posterior Keywords: Gastropexy and anterior wall of the gastric wrap and then passed gently 1 cm above the celiac artery from the denser fi Nissen fundoplication bers of uppermost part of the arcuate ligament. Demographic data, preoperative and postoperative Gastroesophageal reflux assesments of sympthomatic and functional outcomes of patients were recorded. -

Modified Heller´S Esophageal Myotomy Associated with Dor's
Crimson Publishers Research Article Wings to the Research Modified Heller´s Esophageal Myotomy Associated with Dor’s Fundoplication A Surgical Alternative for the Treatment of Dolico Megaesophagus Fernando Athayde Veloso Madureira*, Francisco Alberto Vela Cabrera, Vernaza ISSN: 2637-7632 Monsalve M, Moreno Cando J, Charuri Furtado L and Isis Wanderley De Sena Schramm Department of General Surgery, Brazil Abstracts The most performed surgery for the treatment of achalasia is Heller´s esophageal myotomy associated or no with anti-reflux fundoplication. We propose in cases of advanced megaesophagus, specifically in the dolico megaesophagus, a technical variation. The aim of this study was to describe Heller´s myotomy modified by Madureira associated with Dor´s fundoplication as an alternative for the treatment of dolico megaesophagus,Materials and methods: assessing its effectiveness at through dysphagia scores and quality of life questionnaires. *Corresponding author: proposes the dissection ofTechnical the esophagus Note describing intrathoracic, the withsurgical circumferential procedure and release presenting of it, in the the results most of three patients with advanced dolico megaesophagus, operated from 2014 to 2017. The technique A. V. Madureira F, MsC, Phd. Americas Medical City Department of General extensive possible by trans hiatal route. Then the esophagus is retracted and fixed circumferentially in the Surgery, Full Professor of General pillars of the diaphragm with six or seven point. The goal is at least on the third part of the esophagus, to achieveResults: its broad mobilization and rectification of it; then is added a traditional Heller myotomy. Submission:Surgery At UNIRIO and PUC- Rio, Brazil Published: The mean dysphagia score in pre-op was 10points and in the post- op was 1.3 points (maximum October 09, 2019 of 10 points being observed each between the pre and postoperative 8.67 points, 86.7%) The mean October 24, 2019 hospitalization time was one day. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

The Short Esophagus—Lengthening Techniques
10 Review Article Page 1 of 10 The short esophagus—lengthening techniques Reginald C. W. Bell, Katherine Freeman Institute of Esophageal and Reflux Surgery, Englewood, CO, USA Contributions: (I) Conception and design: RCW Bell; (II) Administrative support: RCW Bell; (III) Provision of the article study materials or patients: RCW Bell; (IV) Collection and assembly of data: RCW Bell; (V) Data analysis and interpretation: RCW Bell; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Reginald C. W. Bell. Institute of Esophageal and Reflux Surgery, 499 E Hampden Ave., Suite 400, Englewood, CO 80113, USA. Email: [email protected]. Abstract: Conditions resulting in esophageal damage and hiatal hernia may pull the esophagogastric junction up into the mediastinum. During surgery to treat gastroesophageal reflux or hiatal hernia, routine mobilization of the esophagus may not bring the esophagogastric junction sufficiently below the diaphragm to provide adequate repair of the hernia or to enable adequate control of gastroesophageal reflux. This ‘short esophagus’ was first described in 1900, gained attention in the 1950 where various methods to treat it were developed, and remains a potential challenge for the contemporary foregut surgeon. Despite frequent discussion in current literature of the need to obtain ‘3 or more centimeters of intra-abdominal esophageal length’, the normal anatomy of the phrenoesophageal membrane, the manner in which length of the mobilized esophagus is measured, as well as the degree to which additional length is required by the bulk of an antireflux procedure are rarely discussed. Understanding of these issues as well as the extent to which esophageal shortening is due to factors such as congenital abnormality, transmural fibrosis, fibrosis limited to the esophageal adventitia, and mediastinal fixation are needed to apply precise surgical technique. -
![Spleen Rupture Complicating Upper Endoscopy in the Medical Literature [3–5]](https://docslib.b-cdn.net/cover/3489/spleen-rupture-complicating-upper-endoscopy-in-the-medical-literature-3-5-863489.webp)
Spleen Rupture Complicating Upper Endoscopy in the Medical Literature [3–5]
E206 UCTN – Unusual cases and technical notes following gastroscopy [3]. To our knowl- edge, only few cases have been reported Spleen rupture complicating upper endoscopy in the medical literature [3–5]. We think that the excessive stretching of spleno-diaphragmatic ligaments and of spleno-peritoneal lateral attachments Fig. 1 Computed during endoscopy and possibly the loca- tomography (CT) scan of abdomen in an 81- tion of most of the stomach in the thoracic year-old woman with cavity had contributed to the spleen rup- generalized weakness, ture [5,6]. Rapid diagnosis in the presence persistent nausea, and of suggestive symptoms of hemodynamic difficulty swallowing, instability and abdominal pain following showing hemoperito- upper endoscopy is life-saving. neum, subcapsular spleen hematoma, and blood around the liver. Endoscopy_UCTN_Code_CPL_1AH_2AJ Competing interests: None F. Jabr1, N. Skeik2 1 Hospital Medicine, Horizon Medical Center, Tennessee, USA 2 Vascular Medicine, Abott Northwestern An 81-year-old woman with history of peritoneum with subcapsular hematoma Hospital, Minneapolis, USA chronic lymphocytic leukemia and recent on the spleen (●" Fig. 1). The patient was diagnosis of Clostridium difficile colitis, diagnosed as having splenic rupture. Ex- and maintained on oral vancomycin, pre- ploratory laparotomy showed large he- References sented for generalized weakness, persis- moperitoneum (about 1500 mL blood), 1 Lopez-Tomassetti Fernandez EM, Delgado Plasencia L, Arteaga González IJ et al. Atrau- tent nausea, and a long history of difficulty subcapsular hematoma of the lateral in- matic rupture of the spleen: experience of swallowing (food hangs in her chest and ferior portion of the spleen, as well as a 10 cases. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
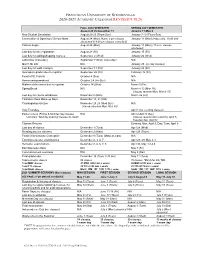
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -

Advances in Flexible Endoscopy
Advances in Flexible Endoscopy Anant Radhakrishnan, DVM KEYWORDS Flexible endoscopy Minimally invasive procedures Gastroduodenoscopy Minimally invasive surgery KEY POINTS Although some therapeutic uses exist, flexible endoscopy is primarily used as a diagnostic tool. Several novel flexible endoscopic procedures have been studied recently and show prom- ise in veterinary medicine. These procedures provide the clinician with increased diagnostic capability. As the demand for minimally invasive procedures continues to increase, flexible endos- copy is being more readily investigated for therapeutic uses. The utility of flexible endoscopy in small animal practice should increase in the future with development of the advanced procedures summarized herein. INTRODUCTION The demand for minimally invasive therapeutic measures continues to increase in hu- man and veterinary medicine. Pet owners are increasingly aware of technology and diagnostic options and often desire the same care for their pet that they may receive if hospitalized. Certain diseases, such as neoplasia, hepatobiliary disease, pancreatic disease, and gastric dilatation–volvulus, can have significant morbidity associated with them such that aggressive, invasive measures may be deemed unacceptable. Even less severe chronic illnesses such as inflammatory bowel disease can be asso- ciated with frustration for the pet owner such that more immediate and detailed infor- mation regarding their pet’s disease may prove to be beneficial. Minimally invasive procedures that can increase diagnostic and therapeutic capability with reduced pa- tient morbidity will be in demand and are therefore an area of active investigation. The author has nothing to disclose. Department of Internal Medicine, Bluegrass Veterinary Specialists 1 Animal Emergency, 1591 Winchester Road, Suite 106, Lexington, KY 40505, USA E-mail address: [email protected] Vet Clin Small Anim 46 (2016) 85–112 http://dx.doi.org/10.1016/j.cvsm.2015.08.003 vetsmall.theclinics.com 0195-5616/16/$ – see front matter Ó 2016 Elsevier Inc. -

2018 - 2019 Days of Rotation Calendar
2018 - 2019 DAYS OF ROTATION CALENDAR Day # Date Rotation Day Type Notes Day # Date Rotation Day Type Notes Saturday, October 13, 2018 Sunday, October 14, 2018 Monday, September 3, 2018 Holiday/Vaca Labor Day 27 Monday, October 15, 2018 Day 3 In Session 1 Tuesday, September 4, 2018 Day 1 In Session 28 Tuesday, October 16, 2018 Day 4 In Session 2 Wednesday, September 5, 2018 Day 2 In Session 29 Wednesday, October 17, 2018 Day 5 In Session 3 Thursday, September 6, 2018 Day 3 In Session 30 Thursday, October 18, 2018 Day 6 In Session 4 Friday, September 7, 2018 Day 4 In Session 31 Friday, October 19, 2018 Day 1 In Session Saturday, September 8, 2018 Saturday, October 20, 2018 Sunday, September 9, 2018 Sunday, October 21, 2018 Monday, September 10, 2018 Day Holiday/Vaca Rosh Hashanah 32 Monday, October 22, 2018 Day 2 In Session 5 Tuesday, September 11, 2018 Day 5 In Session 33 Tuesday, October 23, 2018 Day 3 In Session 6 Wednesday, September 12, 2018 Day 6 In Session 34 Wednesday, October 24, 2018 Day 4 In Session 7 Thursday, September 13, 2018 Day 1 In Session 35 Thursday, October 25, 2018 Day 5 In Session 8 Friday, September 14, 2018 Day 2 In Session 36 Friday, October 26, 2018 Day 6 In Session Saturday, September 15, 2018 Saturday, October 27, 2018 Sunday, September 16, 2018 Sunday, October 28, 2018 9 Monday, September 17, 2018 Day 3 In Session 37 Monday, October 29, 2018 Day 1 In Session 10 Tuesday, September 18, 2018 Day 4 In Session 38 Tuesday, October 30, 2018 Day 2 In Session Wednesday, September 19, 2018 Day Holiday/Vaca Yom Kippur 39 Wednesday, October 31, 2018 Day 3 In Session 11 Thursday, September 20, 2018 Day 5 In Session 40 Thursday, November 1, 2018 Day 4 In Session 12 Friday, September 21, 2018 Day 6 In Session 41 Friday, November 2, 2018 Day 5 In Session Saturday, September 22, 2018 Saturday, November 3, 2018 Sunday, September 23, 2018 Sunday, November 4, 2018 13 Monday, September 24, 2018 Day 1 In Session 42 Monday, November 5, 2018 Day 6 In Session 14 Tuesday, September 25, 2018 Day 2 In Session Tuesday, November 6, 2018 Prof Dev.