Nabalus Bootii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Flora of Burden Hill Forest: a Checklist for a Salem County, New Jersey Landscape
Bartonia No. 69: 20–46, 2016 Flora of Burden Hill Forest: a Checklist for a Salem County, New Jersey Landscape TED GORDON1 AND JOSEPH ARSENAULT2 1Pine Barrens Inventories, 31 Burrs Mill Road, Southampton, NJ 08088 2961 Clark Avenue, Franklinville, NJ 08322 ABSTRACT. We provide a checklist of native and non-native plants growing within a region of Salem County described as Burden Hill Forest. This list is the result of more than a dozen surveys that we conducted between 2001 and 2012. 530 distinct taxa (106 families, 303 genera, 520 species, and 10 varieties) were identified within the forests and meadows found between the villages of Alloway and Stow Creek. Included are habitat descriptions and site locations for plants of special interest. A landscape description is provided that distinguishes this forest and its flora from other New Jersey Outer Coastal Plain forests. INTRODUCTION Salem County, New Jersey is not a region one thinks of as supporting large contiguous forests. Cleared and farmed since the late 1600s (Smith 1877), Salem County is best known for vistas of bucolic fields and expansive tidal marshes. Early colonial farmers learned that the soils between the Delaware River and Delaware Bay were excellent for crops and livestock. Soon after the Finns, Swedes, and English gained a foothold, the county’s forests yielded to the progress of the ax and stock animals, “Their lands and meadows are rich, and production of any kind, natural to the climate, plenty” (Smith 1877). Few forests survived the initial clearing, resulting in a landscape that was mapped by the 1880s without any woodland larger than 50 acres (Vermeule 1884, 1900). -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Nabalus Racemosus (Michx.) Hook. Glaucous White Lettuce
New England Plant Conservation Program Nabalus racemosus (Michx.) Hook. Glaucous white lettuce Conservation and Research Plan for New England Prepared by: Lisa St. Hilaire Ecologist 14 Prospect St. Augusta, Maine 04339 USA For: New England Wild Flower Society 180 Hemenway Road Framingham, Massachusetts 01701 USA 508/877-7630 e-mail: [email protected] • website: www.newfs.org Approved, Regional Advisory Council, December 2003 1 SUMMARY Nabalus racemosus (Michx.) Hook., glaucous white lettuce, is a perennial member of the Asteraceae or composite family. It is considered globally secure (G5), but in New England, it is known only from northern Maine, primarily along the St. John River. There are also several occurrences along the Aroostook River. Ice scour and flooding are common annual disturbances on these rivers. Many of the N. racemosus populations were discovered during survey efforts for Pedicularis furbishiae (Furbish’s lousewort), and both species, as well as many other rarities, may be found at some sites. In other parts of the country, N. racemosus grows in prairie communities. There are currently 31 extant occurrences in Maine, 28 of these along the St. John River, and three on the Aroostook River. There are four historic occurrences, all on the Aroostook River, and one extirpated population on the Aroostook River. Nabalus racemosus is a species of Special Concern in Maine, where it is ranked S3. Other nearby areas from which it is recorded include New Brunswick (S3), Nova Scotia (S1), Newfoundland Island (S1S2), Labrador (SR), Quebec (SR), Vermont (SR), New Jersey (SH), New York (SX), and Pennsylvania (SX). Little is known regarding the biology of Nabalus racemosus. -

Botanical Inventory Report
Botanical Inventory Mason Quarry Conservation Area Mason, New Hampshire Erin Schaeffer New England Wild Flower Society © 2013 Prepared by 180 Hemenway Road Framingham, MA 01701 508-877-7630 www.newfs.org Amanda Weise John Burns Conducted in 2013 This report was produced for the Town of Mason, Conservation Commission This project was made possible by a generous donation from Catherine Schwenk TABLE OF CONTENTS INTRODUCTION ........................................................................................................................... 4 METHODS ...................................................................................................................................... 6 RESULTS ........................................................................................................................................ 7 PLANT SPECIES ............................................................................................................... 7 PLANT IDENTIFICATIONS ............................................................................................ 7 NATURAL COMMUNITIES ............................................................................................ 8 DISCUSSION .................................................................................................................................. 8 COUNTY RECORDS ........................................................................................................ 9 RARE PLANTS ................................................................................................................. -

Mississippi Natural Heritage Program Special Plants - Tracking List -2018
MISSISSIPPI NATURAL HERITAGE PROGRAM SPECIAL PLANTS - TRACKING LIST -2018- Approximately 3300 species of vascular plants (fern, gymnosperms, and angiosperms), and numerous non-vascular plants may be found in Mississippi. Many of these are quite common. Some, however, are known or suspected to occur in low numbers; these are designated as species of special concern, and are listed below. There are 495 special concern plants, which include 4 non- vascular plants, 28 ferns and fern allies, 4 gymnosperms, and 459 angiosperms 244 dicots and 215 monocots. An additional 100 species are designated “watch” status (see “Special Plants - Watch List”) with the potential of becoming species of special concern and include 2 fern and fern allies, 54 dicots and 44 monocots. This list is designated for the primary purposes of : 1) in environmental assessments, “flagging” of sensitive species that may be negatively affected by proposed actions; 2) determination of protection priorities of natural areas that contain such species; and 3) determination of priorities of inventory and protection for these plants, including the proposed listing of species for federal protection. GLOBAL STATE FEDERAL SPECIES NAME COMMON NAME RANK RANK STATUS BRYOPSIDA Callicladium haldanianum Callicladium Moss G5 SNR Leptobryum pyriforme Leptobryum Moss G5 SNR Rhodobryum roseum Rose Moss G5 S1? Trachyxiphium heteroicum Trachyxiphium Moss G2? S1? EQUISETOPSIDA Equisetum arvense Field Horsetail G5 S1S2 FILICOPSIDA Adiantum capillus-veneris Southern Maidenhair-fern G5 S2 Asplenium -

Dispersal Effects on Species Distribution and Diversity Across Multiple Scales in the Southern Appalachian Mixed Mesophytic Flora
DISPERSAL EFFECTS ON SPECIES DISTRIBUTION AND DIVERSITY ACROSS MULTIPLE SCALES IN THE SOUTHERN APPALACHIAN MIXED MESOPHYTIC FLORA Samantha M. Tessel A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Ecology in the Curriculum for the Environment and Ecology. Chapel Hill 2017 Approved by: Peter S. White Robert K. Peet Alan S. Weakley Allen H. Hurlbert Dean L. Urban ©2017 Samantha M. Tessel ALL RIGHTS RESERVED ii ABSTRACT Samantha M. Tessel: Dispersal effects on species distribution and diversity across multiple scales in the southern Appalachian mixed mesophytic flora (Under the direction of Peter S. White) Seed and spore dispersal play important roles in the spatial distribution of plant species and communities. Though dispersal processes are often thought to be more important at larger spatial scales, the distribution patterns of species and plant communities even at small scales can be determined, at least in part, by dispersal. I studied the influence of dispersal in southern Appalachian mixed mesophytic forests by categorizing species by dispersal morphology and by using spatial pattern and habitat connectivity as predictors of species distribution and community composition. All vascular plant species were recorded at three nested sample scales (10000, 1000, and 100 m2), on plots with varying levels of habitat connectivity across the Great Smoky Mountains National Park. Models predicting species distributions generally had higher predictive power when incorporating spatial pattern and connectivity, particularly at small scales. Despite wide variation in performance, models of locally dispersing species (species without adaptations to dispersal by wind or vertebrates) were most frequently improved by the addition of spatial predictors. -

5. Tribe CICHORIEAE 菊苣族 Ju Ju Zu Shi Zhu (石铸 Shih Chu), Ge Xuejun (葛学军); Norbert Kilian, Jan Kirschner, Jan Štěpánek, Alexander P
Published online on 25 October 2011. Shi, Z., Ge, X. J., Kilian, N., Kirschner, J., Štěpánek, J., Sukhorukov, A. P., Mavrodiev, E. V. & Gottschlich, G. 2011. Cichorieae. Pp. 195–353 in: Wu, Z. Y., Raven, P. H. & Hong, D. Y., eds., Flora of China Volume 20–21 (Asteraceae). Science Press (Beijing) & Missouri Botanical Garden Press (St. Louis). 5. Tribe CICHORIEAE 菊苣族 ju ju zu Shi Zhu (石铸 Shih Chu), Ge Xuejun (葛学军); Norbert Kilian, Jan Kirschner, Jan Štěpánek, Alexander P. Sukhorukov, Evgeny V. Mavrodiev, Günter Gottschlich Annual to perennial, acaulescent, scapose, or caulescent herbs, more rarely subshrubs, exceptionally scandent vines, latex present. Leaves alternate, frequently rosulate. Capitulum solitary or capitula loosely to more densely aggregated, sometimes forming a secondary capitulum, ligulate, homogamous, with 3–5 to ca. 300 but mostly with a few dozen bisexual florets. Receptacle naked, or more rarely with scales or bristles. Involucre cylindric to campanulate, ± differentiated into a few imbricate outer series of phyllaries and a longer inner series, rarely uniseriate. Florets with 5-toothed ligule, pale yellow to deep orange-yellow, or of some shade of blue, including whitish or purple, rarely white; anthers basally calcarate and caudate, apical appendage elongate, smooth, filaments smooth; style slender, with long, slender branches, sweeping hairs on shaft and branches; pollen echinolophate or echinate. Achene cylindric, or fusiform to slenderly obconoidal, usually ribbed, sometimes compressed or flattened, apically truncate, attenuate, cuspi- date, or beaked, often sculptured, mostly glabrous, sometimes papillose or hairy, rarely villous, sometimes heteromorphic; pappus of scabrid [to barbellate] or plumose bristles, rarely of scales or absent. -

Additions to the New Flora of Vermont
Gilman, A.V. Additions to the New Flora of Vermont. Phytoneuron 2016-19: 1–16. Published 3 March 2016. ISSN 2153 733X ADDITIONS TO THE NEW FLORA OF VERMONT ARTHUR V. GILMAN Gilman & Briggs Environmental 1 Conti Circle, Suite 5, Barre, Vermont 05641 [email protected] ABSTRACT Twenty-two species of vascular plants are reported for the state of Vermont, additional to those reported in the recently published New Flora of Vermont. These are Agrimonia parviflora, Althaea officinalis , Aralia elata , Beckmannia syzigachne , Bidens polylepis , Botrychium spathulatum, Carex panicea , Carex rostrata, Eutrochium fistulosum , Ficaria verna, Hypopitys lanuginosa, Juncus conglomeratus, Juncus diffusissimus, Linum striatum, Lipandra polysperma , Matricaria chamomilla, Nabalus racemosus, Pachysandra terminalis, Parthenocissus tricuspidata , Ranunculus auricomus , Rosa arkansana , and Rudbeckia sullivantii. Also new are three varieties: Crataegus irrasa var. irrasa , Crataegus pruinosa var. parvula , and Viola sagittata var. sagittata . Three species that have been reported elsewhere in 2013–2015, Isoetes viridimontana, Naias canadensis , and Solidago brendiae , are also recapitulated. This report and the recently published New Flora of Vermont (Gilman 2015) together summarize knowledge of the vascular flora of Vermont as of this date. The New Flora of Vermont was recently published by The New York Botanical Garden Press (Gilman 2015). It is the first complete accounting of the vascular flora of Vermont since 1969 (Seymour 1969) and adds more than 200 taxa to the then-known flora of the state. However, the manuscript for the New Flora was finalized in spring 2013 and additional species are now known: those that have been observed more recently, that have been recently encountered (or re-discovered) in herbaria, or that were not included because they were under study at the time of finalization. -

Plants of Southeastern Alaska
Proceedings of the Iowa Academy of Science Volume 25 Annual Issue Article 38 1918 Plants of Southeastern Alaska J. P. Anderson Let us know how access to this document benefits ouy Copyright ©1918 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Anderson, J. P. (1918) "Plants of Southeastern Alaska," Proceedings of the Iowa Academy of Science, 25(1), 427-449. Available at: https://scholarworks.uni.edu/pias/vol25/iss1/38 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Anderson: Plants of Southeastern Alaska PLANTS OF SOUTHEASTERN ALASKA. J. P. ANDERSON. The following list of species is based on collection and observa tions by the writer except in the case of a few grasses and sedges from Sitka of which specimens are in the herbarium of the U. S. Agricultural Experiment Station at that place. Proper facilities for the determination of all the species not being at hand, sets were sent away for determination. The Sitka collections were sent to the U. S. National Herbarium while the plants from Juneau were sent to the New York Botanical Garden where they were determined by Dr. P. A. Rydberg. In regard to the localities mentioned the following notes may prove helpful. Sitka is situated on the west or seaward side of Baranoff island in latitude 75° 3' N. -

Nabalus Aspera (Asteraceae) in Louisiana
Holmes, W.C. and J.R. Singhurst. 2012. Nabalus aspera (Asteraceae) in Louisiana. Phytoneuron 2012-60: 1–4. Published 9 July 2012. ISSN 2153 733X NABALUS ASPERA (ASTERACEAE) IN LOUISIANA Walter C. Holmes Department of Biology Baylor University Waco, Texas 76798-7388 U.S.A . [email protected] Jason R. Singhurst Wildlife Diversity Program Texas Parks and Wildlife Department 4200 Smith School Road Austin, Texas 78704 U.S.A. [email protected] ABSTRACT The occurrence of Nabalus (Prenanthes) asper (Asteraceae) is documented in Louisiana. It was collected by Josiah Hale sometime before 1843, probably in Rapides Parish in some isolated prairie that no longer exists. KEY WORDS : Asteraceae, Compositae, Nabalus , Prenanthes , Louisiana, Josiah Hale, isolated prairies In the course of studying the vascular flora of Texas, we occasionally discover information concerning the flora of adjoining states. In this instance, a historic collection of Nabalus asper (Michx.) Torrey & A. Gray from Louisiana permits documentation of the species in that state, something, until now, never done. This particular study originated with our discovery, study, and report of the species in Texas, under the name Prenanthes aspera Michx. (Singhurst & Holmes 2010). Following is a short history, with commentary, of Nabalus asper in Louisiana. The initial report of the species in Louisiana was by Torrey and Gray (1843), who proposed the new combination Nabalus asper , based upon Michaux’s Prenanthes aspera (spelling of the epithet varies by gender). In this work, Torrey and Gray used the exclamation point (!) “after the manner in which it is employed by De Candolle and other modern botanists, to indicate that we have seen an authentic specimen of the author, or from the location cited.” Thus the distribution was given as this: “Dry barrens and prairies of Ohio! Indiana! Louisiana! and upper Missouri!” No other data was presented. -
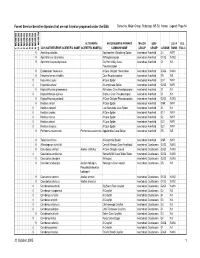
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR