Some Aspects of Camouflage in Animals By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heathland 700 the Park & Poor's Allotment Species List
The Park & Poor's Allotment Bioblitz 25th - 26th July 2015 Common Name Scientific Name [if known] Site recorded Fungus Xylaria polymorpha Dead Man's Fingers Both Amanita excelsa var. excelsa Grey Spotted Amanita Poor's Allotment Panaeolus sp. Poor's Allotment Phallus impudicus var. impudicus Stinkhorn The Park Mosses Sphagnum denticulatum Cow-horn Bog-moss Both Sphagnum fimbriatum Fringed Bog-moss The Park Sphagnum papillosum Papillose Bog-moss The Park Sphagnum squarrosum Spiky Bog-moss The Park Sphagnum palustre Blunt-leaved Bog-moss Poor's Allotment Atrichum undulatum Common Smoothcap Both Polytrichum commune Common Haircap The Park Polytrichum formosum Bank Haircap Both Polytrichum juniperinum Juniper Haircap The Park Tetraphis pellucida Pellucid Four-tooth Moss The Park Schistidium crassipilum Thickpoint Grimmia Poor's Allotment Fissidens taxifolius Common Pocket-moss The Park Ceratodon purpureus Redshank The Park Dicranoweisia cirrata Common Pincushion Both Dicranella heteromalla Silky Forklet-moss Both Dicranella varia Variable Forklet-moss The Park Dicranum scoparium Broom Fork-moss Both Campylopus flexuosus Rusty Swan-neck Moss Poor's Allotment Campylopus introflexus Heath Star Moss Both Campylopus pyriformis Dwarf Swan-neck Moss The Park Bryoerythrophyllum Red Beard-moss Poor's Allotment Barbula convoluta Lesser Bird's-claw Beard-moss The Park Didymodon fallax Fallacious Beard-moss The Park Didymodon insulanus Cylindric Beard-moss Poor's Allotment Zygodon conoideus Lesser Yoke-moss The Park Zygodon viridissimus Green Yoke-moss -

Elpenor 2010-2015
Projet ELPENOR MACROHETEROCERES DU CANTON DE GENEVE : POINTAGE DES ESPECES PRESENTES Résultats des prospections 2010-2015 Pierre BAUMGART & Maxime PASTORE « Voilà donc les macrohétérocéristes ! Je les imaginais introvertis, le teint blafard, disséquant, cataloguant, épinglant. Ils sont là, enjoués, passionnés, émerveillés par les trésors enfouis des nuits genevoises ! » Blaise Hofman, « La clé des champs » SOMMAIRE • ELPENOR ? 2 • INTRODUCTION 3 • PROTOCOLE DE CHASSE 4 • FICHE D’OBSERVATIONS 4 • MATÉRIEL DE TERRAIN 5 • SITES PROSPECTÉS 7 - 11 • ESPÈCES OBSERVÉES 2010 – 2015 13 (+ 18 p. hors-texte) • ESPECES OBSERVEES CHAQUE ANNEE 13’ • ECHANTILLONNAGE D’ESPECES 14 • CHRONOLOGIE DES OBSERVATIONS REMARQUABLES 15 – 17 • ESPECES A RECHERCHER 18-20 • AUTRES VISITEURS… 21 • PUBLICATIONS 22 (+ 4 p. hors-texte) • ESPECES AJOUTEES A LA LISTE 23 • RARETÉS 24 • DISCUSSION 25 - 26 • PERSPECTIVES 27 • CHOIX DE CROQUIS DE TERRAIN 29 - 31 • COUPURES DE PRESSE 33 - 35 • ALBUM DE FAMILLE 36 • REMERCIEMENTS 37 • BIBLIOGRAPHIE & RESSOURCES INTERNET 38 – 39 1 ELPENOR ? Marin et compagnon d'Ulysse à son retour de la guerre de Troie, Elpenor (en grec Ἐλπήνορος , « homme de l'espoir ») est de ceux qui, sur l'île d'Aenea, furent victimes de la magicienne Circé et transformés en pourceaux jusqu'à ce qu'Ulysse, qui avait été préservé des enchantements de la magicienne grâce à une herbe offerte par le dieu Hermès, la contraigne à redonner à ses compagnons leur forme humaine. Lors de la fête qui s’ensuivit, Elpenor, pris de boisson, s'endormit sur la terrasse de la demeure de Circé, et, réveillé en sursaut, se tua en tombant du toit. Lorsqu'il descendit aux Enfers pour consulter le devin Tirésias, Ulysse croisa l’ombre de son défunt compagnon, à laquelle il promit une sépulture honorable. -

Научные Исследования В Зоологических Парках Scientific Research in Zoological Parks
ДЕПАРТАМЕНТ КУЛЬТУРЫ ГОРОДА МОСКВЫ DEPARTMENT OF CULTURE OF MOSCOW ЕВРОАЗИАТСКАЯ РЕГИОНАЛЬНАЯ АССОЦИАЦИЯ ЗООПАРКОВ И АКВАРИУМОВ EUROASIAN REGIONAL ASSOCIATION OF ZOOS AND AQUARIA ГАУ «МОСКОВСКИЙ ЗООПАРК» MOSCOW ZOO Научные исследования в зоологических парках Scientific research in zoological parks Выпуск 33 Volume 33 Москва КолорВитрум 2018 УДК: 59:[591.1+591.2+591.5]:502 ОТ РЕДАКЦИИ ББК 28.6л6 Н 34 Сборник «Научные исследования в зоологических парках» выходит в конце года. В ближайший выпуск попадают статьи, окончательный (не требующий правки) вариант Н 34 Научные исследования в зоологических парках. Выпуск 33. Сборник научных которых получен до 1 июня текущего года. исследований. — М. : ООО «КолорВитрум», 2018. — 162с. Для публикации в сборнике принимаются работы, содержащие: результаты Библ.: 184 назв.; табл.: 15; рис.: 36; эл. рес.: 13. научных и научно-практических исследований, выполненных в зоопарках или касающихся особенностей биологии и поведения животных, важных для содержания их в неволе; аналитические статьи; обзоры литературы; описание и анализ интересных случаев зоопарковской практики; материалы по истории зоопарковского дела. Кроме того, мы публикуем информационные материалы о семинарах, конференциях и т. п. мероприятиях по профильной тематике. К публикации не принимаются материалы, не относящиеся к проблематике содержания животных в неволе. Объем принимаемых Дорогие коллеги! материалов: краткие сообщения и описания отдельных событий — до 5 стр.; Мы предлагаем вашему вниманию очередной 33 выпуск сборника Московского зоопарка «Научные аналитические и обзорные статьи и обзоры литературы — до 30 стр.; проблемные исследования в зоологических парках», посвященный различным аспектам зоопарковской деятельности. и критические статьи — до 20 стр. С содержанием этого и предыдущих сборников можно ознакомиться на сайте Московского зоопарка Присылаемые рукописи принимаются в электронном виде, в формате Winword, www.moscowzoo.ru в разделе «Специалистам. -

Harper's Island Wetlands Butterflies & Moths (2020)
Introduction Harper’s Island Wetlands (HIW) nature reserve, situated close to the village of Glounthaune on the north shore of Cork Harbour is well known for its birds, many of which come from all over northern Europe and beyond, but there is a lot more to the wildlife at the HWI nature reserve than birds. One of our goals it to find out as much as we can about all aspects of life, both plant and animal, that live or visit HIW. This is a report on the butterflies and moths of HIW. Butterflies After birds, butterflies are probably the one of the best known flying creatures. While there has been no structured study of them on at HIW, 17 of Ireland’s 33 resident and regular migrant species of Irish butterflies have been recorded. Just this summer we added the Comma butterfly to the island list. A species spreading across Ireland in recent years possibly in response to climate change. Hopefully we can set up regular monitoring of the butterflies at HIW in the next couple of years. Butterfly Species Recorded at Harper’s Island Wetlands up to September 2020. Colias croceus Clouded Yellow Pieris brassicae Large White Pieris rapae Small White Pieris napi Green-veined White Anthocharis cardamines Orange-tip Lycaena phlaeas Small Copper Polyommatus icarus Common Blue Celastrina argiolus Holly Blue Vanessa atalanta Red Admiral Vanessa cardui Painted Lady Aglais io Peacock Aglais urticae Small Tortoiseshell Polygonia c-album Comma Speyeria aglaja Dark-green Fritillary Pararge aegeria Speckled Wood Maniola jurtina Meadow Brown Aphantopus hyperantus Ringlet Moths One group of insects that are rarely seen by visitors to HIW is the moths. -

Moths Recorded at Simpson's Fromus Valley Recorder: Pete Rowberry
Suffolk Flora Preservation Trust Moths recorded at Simpson's Fromus Valley Recorder: Pete Rowberry Common Name Binomial Occurrences No of days recorded Barred Sallow Xanthia aurago 3 1 Barred Yellow Cidaria fulvata 4 1 Beautiful Hook Tip Laspeyria flexula 2 1 Bird Cherry Ermine Yponomeuta evonymella 1 1 Blood Vein Timandra comae 1 1 Buff Ermine Spilosoma luteum 2 1 Buff Tip Phalera bucephala 3 1 Burnished Brass Diachrysia chrysitis 2 1 Chinese Character Cillix glaucata 3 3 Clouded Border Lomaspilis marginata 2 1 Common Carpet Epirrhoe alternata 1 1 Common Footman Eilema lurideola 3 2 Common Rustic Mesapamea secalis 4 2 Common Wainscot Mythimna pallens 4 1 Confused Apamea furva 1 1 Copper Underwing spp. Amphipyra pyramidea 1 1 Dark Arches Apamea monoglypha 4 2 Dark Swordgrass Agrotis ipsilon 1 1 Dingy Footman Eilema griseola 5 2 Double Square Spot Xestia triangulum 2 2 Dunbar Cosmia trapezina 1 1 Dusky Sallow Eremobia ochroleuca 3 2 Flame Shoulder Ochropleura plecta 4 2 Flounced Rustic Luperina testacea 1 1 Garden Carpet Xanthorhae fluctuata 1 1 Green Carpet Colostygia pectinataria 1 1 Green Pug Pasiphila rectangulata 1 1 Grey Tortrix Cmephasia stephensiana 1 1 Hebrew Character Orthosia gothica 1 1 Iron Prominent Notodonta dromedarius 1 1 Large Yellow Underwing Noctua pronuba 30 5 Latticed Heath Chiasmia clathrata 1 1 Lunar Underwing Omphaloscelis lumosa 2 2 Magpie Abraxas grossulariata 2 2 Mother of Pearl Pleuoptya ruralis Mottled Beauty Alcis repandata 2 2 Muslin Moth Diaphora mendica 1 1 Pebble Prominent Notodonta ziczac 1 1 Peppered -
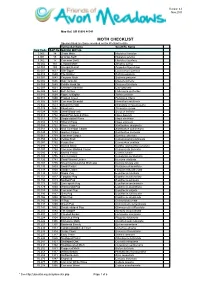
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

NUMBER 13 – August 2010 Wallblings
Herald Moth, Pamber © Paul Sterry NUMBER 13 – August 2010 Wallblings... Welcome to the 13th Hantsmoths newsletter, delayed more than usual due to my annual foray across the Channel to pastures warmer where the moths are more exotic and therefore covers July and August together. Thanks to Tim for putting the bulk of this missive together in my absence. Please keep the news coming in, either to me directly at mike AT hantsmoths.org.uk, or via the Hantsmoths yahoogroup. Until the next time, Good mothing! Mike --------------- 1 Request for info: Cnephasia pumicana – new to British (and Hampshire) list For those who don’t get the Ent.Rec., the following is lifted directly from Entomologist’s Rec. J. Var. 122 (2010) (authored by JRL & DJLA), announcing a new species to the British list. “Moths of the genus Cnephasia Curtis are notoriously difficult to identify from their external morphology. It is often necessary to make a genitalia preparation to be sure of the identity. Whilst examining such preparations JRL observed a striking difference between two males of this complex. The paper by Chambon & Genestier (1980) illustrated and described these differences and suggested we had two species under the name of pasiuana . C. pumicana had been sunk into the synonymy of C. pasiuana by Razowski (1989), although Karsholt & Razowski (1996) mention that this synonymy was not accepted by Jaroś (1993). It was also included as a distinct species in Novak & Liška (1997), Szabóky et al. (2002) and in Aarvik (2004). It remains listed as a synonym of pasiuana in the popular work by Razowski (2002). -

In Istria in Autumn
Go Slow…. In Istria in Autumn Naturetrek Tour Report 1 - 8 October 2019 Althea cannabina Flame Brocade Spiranthes spiralis Monkodonja Report and images by Paul Tout & Paul Harmes Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Go Slow…. In Istria in Autumn Tour participants: Paul Tout and Paul Harmes (leaders) with 14 Naturetrek clients Day 1 Tuesday 1st October Trieste Airport - Istarske Toplice (our hotel accommodation) Paul Harmes met up with the group at Stansted. Arriving at Trieste airport just after 5pm, we passed through passport control, collected our luggage and moved out into the Arrivals hall where we were met by Paul Tout. We were soon on our way towards Istria, passing through the very attractive historic centre of Trieste and on towards Koper-Capodistria, the main port in Slovenia. At the crossroads of Europe where the three main language groups meet (Romance, Slav and Germanic), the area is an ethnic mix with large areas of bi- (and even tri-) lingualism, so place names are usually hyphenated, made up of two languages. The weather was fine, sunny and warm. We took the road for the centre of Trieste along the Costiera. but no Alpine Swifts were visible. They nest in a colony on the cliffs beside the road and they continue to visit the colonies until mid-October but evidently, they were feeding high up in the fine weather. Passing through the centre of Trieste, the city looked very fine in the evening light, with its Viennese-style waterfront and main square, Piazza Unita d’Italia, the only one in Italy that opens onto the sea. -

Moths on Slepe Heath 2017 – 2020
Moths of Poole Harbour is a project of Birds of Poole Harbour Moths on Slepe Heath 2017 – 2020 The ‘Moths of Poole Harbour’ (MoPH) project was set up in 2017 to gain knowledge of moth species occurring in Poole Harbour, Dorset, their distribution, abundance and to some extent, their habitat requirements. The study area shares the same boundaries as the Birds of Poole Harbour (BoPH) project. Birds of Poole Harbour recording area Slepe Heath is a large expanse of lowland heathland contiguous with Hartland Moor and forming an integral part of the Purbeck Heaths National Nature Reserve established in 2020. The site is owned and managed by the National Trust. The site has undergone extensive pine removal within the last decade or so and has a variety of heathland types within it. Extensive dry heathland slopes are bordered to the north by a fringe of damper communities. To the western edge a marshy area is fringed by secondary woodland. Slepe Heath was initially visited following discussions with Mark Parsons at Butterfly Conservation. This site is the known stronghold for the seriously declined Speckled Footman, which became a target species for the project. MoPH visited the heath regularly from mid-July 2017. One of the target species for the Plantlife led ‘Back from the Brink’ Project is the Purbeck Mason Wasp whose larvae feed on the micro-moth Acleris hyemana larvae. MoPH targeted the adult moth in early spring 2018 and 2019 in two of the known sites for Purbeck Mason Wasp on Slepe Heath. Three main areas were regularly visited. -

REPORT on APPLES – Fruit Pathway and Alert List
EU project number 613678 Strategies to develop effective, innovative and practical approaches to protect major European fruit crops from pests and pathogens Work package 1. Pathways of introduction of fruit pests and pathogens Deliverable 1.3. PART 5 - REPORT on APPLES – Fruit pathway and Alert List Partners involved: EPPO (Grousset F, Petter F, Suffert M) and JKI (Steffen K, Wilstermann A, Schrader G). This document should be cited as ‘Wistermann A, Steffen K, Grousset F, Petter F, Schrader G, Suffert M (2016) DROPSA Deliverable 1.3 Report for Apples – Fruit pathway and Alert List’. An Excel file containing supporting information is available at https://upload.eppo.int/download/107o25ccc1b2c DROPSA is funded by the European Union’s Seventh Framework Programme for research, technological development and demonstration (grant agreement no. 613678). www.dropsaproject.eu [email protected] DROPSA DELIVERABLE REPORT on Apples – Fruit pathway and Alert List 1. Introduction ................................................................................................................................................... 3 1.1 Background on apple .................................................................................................................................... 3 1.2 Data on production and trade of apple fruit ................................................................................................... 3 1.3 Pathway ‘apple fruit’ ..................................................................................................................................... -

Moth Survey 2010
Moth Survey at Suffield’s if not now, when wood Dusk – 1am, Saturday 17th July Conducted by the Norfolk Moth Group (www.norfolkmoths.co.uk) Moths are closely related to butterflies. Only around 60 species of butterfly are seen regularly in the UK, but there are around 2,500 species of moths. They are one of the most diverse animal groups on the planet. Unfortunately there as been a serious decline in moth numbers. The Norfolk Moth Group kindly undertook a survey at the wood, such surveys help to improve the knowledge and conservation of these creatures. Please see below for their results # B&F Common Taxon Specimens National Status Foodplant 1 0014 Ghost Moth Hepialus humuli + Resident. Common. Grasses, Herbaceous plants. 2 0116 Stigmella lapponica 1 Common (<100 Norfolk records) Birch. 3 0170 Five-spot Burnet Zygaena trifolii + Very Local in Norfolk. Greater and Common Bird's-foot-trefoil. 4 0464 Diamond-back Moth Plutella xylostella + Migrant Brassica spp. or Cruciferae. 5 0937 Hook-marked Straw Moth Agapeta hamana + Common Thistle. 6 0977 Large Fruit-tree Tortrix Archips podana + Common Deciduous trees. 7 0993 Cyclamen Tortrix Clepsis spectrana + Common Many Herbaceous plants. 8 1076 Celypha lacunana + Common Herbaceous plants. 9 1201 Eucosma cana + Common Thistle and Black Knapweed. 10 1302 Yellow Satin Veneer Crambus perlella + Common Grasses. 11 1304 Pearl Veneer Agriphila straminella + Common Grasses. 12 1331 Water Veneer Acentria ephemerella + Common aquatic plants. Pondweeds and Canadian waterweed. 13 1334 Scoparia ambigualis + Common Mosses. 14 1376 Small Magpie Eurrhypara hortulata + Common Nettle, woundworts, horehounds, bindweeds. 15 1388 Udea lutealis 1 Common Herbaceous plants. -

Butterfly Conservation Upper Thames Branch Moth Sightings Archive - July to December 2012
Butterfly Conservation Upper Thames Branch Moth Sightings Archive - July to December 2012 MOTH SPECIES COUNT FOR 2012 = 946 ~ Friday 25th January 2013 ~ Andy King sent the following: "Peter Hall has identified a number of moths for me and just one of them is of particular note for your site: A Coleophora currucipennella flew into my trap on 23 July 2012 at Philipshill Wood, Bucks. This was a small, brownish unprepossessing thing. Its significance is that it was only the second Bucks record for this proposed Red Data Book 3 species. " ~ Tuesday 8th January 2013 ~ 05/01/13 - Dave Wilton sent the following report: "On 5th January Peter Hall completed the final dissections of difficult moths from me for 2012 and the following can now be added to the year list: Maple Pug (Westcott 8th August), Acompsia cinerella (Steps Hill 14th August), Agonopterix nervosa (Calvert 9th September), Anacampsis blattariella (Finemere Wood 19th August), Caryocolum fraternella (Calvert 12th August), Coleophora albitarsella (Westcott 10th August), Coleophora versurella (Ivinghoe Beacon 9th August), Cosmiotes stabilella (Calvert 17th August), Depressaria badiella (Calvert 12th August), Depressaria chaerophylli (Ivinghoe Beacon 3rd September), Depressaria douglasella (Ivinghoe Beacon 3rd August), Monochroa lutulentella (Finemere Wood 1st September), Oegoconia quadripuncta (Ivinghoe Beacon 9th August), Phyllonorycter oxyacanthae (Westcott 18th August), Scoparia basistrigalis (Calvert 12th August), Stigmella obliquella (Finemere Wood 19th August), Stigmella salicis (private wood near Buckingham 20th August) & Stigmella samiatella (Finemere Wood 17th July). Thankyou Peter!" ~ Friday 7th December 2012 ~ Dave Wilton sent this update: "On 20th November here at Westcott, Bucks my garden actinic trap managed Caloptilia rufipennella (1), Acleris schalleriana (1), an as yet unconfirmed Depressaria sp.