Conjugate Additions and Transposition of the Allylic Alcohols of Enol Ethers of 1, 2-Cyclohexanedione
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mint and Mint Oil Profile New York State Integrated Pest Management Cornell Cooperative Extension Program
http://hdl.handle.net/1813/56133 Mint and Mint Oil Profile New York State Integrated Pest Management Cornell Cooperative Extension Program Mint and Mint Oil Profile Active Ingredient Eligible for Minimum Risk Pesticide Use Brian P. Baker and Jennifer A. Grant New York State Integrated Pest Management, Cornell University, Geneva NY Label Display Names: Cornmint, Cornmint oil, U.S. EPA PC Code: 128800 (Peppermint is also Spearmint, Spearmint oil cross referenced under the listing for mint and mint oil) Active Components: Carvone, Menthol, pulegone, limonene, linarin, pinene, piperitone, linalool, and CA DPR Chem Code: Not Found various other terpenoids, alcohols, esters, and hydrocarbons Other Names: Cornmint oil, spearmint oil, men- thol oil, spearmint terpenes CAS Registry #s: 8008-79-5 (Spearmint oil) Other Codes: 68917-18-0 (Cornmint oil) Cornmint oil—FEMA: 4219; EINECS: 290-058-5; HT: None (Cornmint and Spearmint) 3301.25 Spearmint oil—FEMA: 3032; EINECS: 283-656- HT: 3301.25; RTECS: WG7360000 Summary: Mint and mint oils are a class of active ingredients derived from selected members of the plant genus Mentha. Primary sources include cornmint, peppermint, and spearmint. Active substances contained in the plants and essential oils in this class include menthol, carvone, and various other ter- penoids. Peppermint and spearmint are commonly used food ingredients; cornmint is used as a food ingredient in some Asian cuisines and is a major source of food-grade menthol used as a flavoring agent. As a pesticide, mint and mint oils work primarily through non-toxic modes of action as repellents, but also have anti-microbial properties. Peppermint is covered in a separate profile. -

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance. -
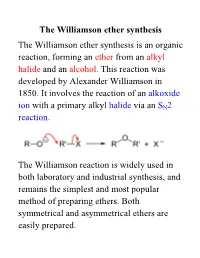
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

United States Patent Patented Dec
Mice 3,293,301 United States Patent Patented Dec. 20, 1966 1 2 tional acid per mole of carvoxime at the beginning of the 3,293,301 hydrolysis. Phosphoric acid is the preferred additional PREPARATION OF CARVONE acid. John M. Derfer, Jacksonville, Bernard J. Kane, Atlantic Also, by using an acceptor for the hydroxylamine it is Beach, and Donald G. Young, Jacksonville, Fla., as not necessary to remove the carvone during the hydrolysis signors to The Glidden Company, Cleveland, Ohio, a as in Reitsema, since there is an equilibrium between corporation of Ohio No Drawing. Filed Apr. 7, 1964, Ser. No. 358,051 carvoxime and its hydrolysis products, carvone and hy- ' 13 Claims. (Cl. 260-—-587) droxylamine, and the reaction can be carried to comple tion before any recovery of the carvone is required. The present invention relates to the preparation of We have found that the objects of the invention can be carvone. achieved when the reactions are carried ‘out in a suitable 'Carvone is an important constituent of many essential solvent such as a lower aliphatic alkanol. The preferred oils. About 65% of spearmint oil is l-carvone and d solvent is isopropyl alcohol. But any other solvent for carvone is the chief constituent of caraway and dill seed carvoxime and carvone which is water soluble, nonreac oils. 15 tive with the reacting materials, and which has a boiling The preparation of l-carvone from d-limonene has been point of at least about 75° C, or higher can be used. described by Bordenca et al., Ind. and Eng. -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity. -

The Generation and Reactivity of Organozinc Carbenoids
The Generation and Reactivity of Organozinc Carbenoids A Thesis Presented by Caroline Joy Nutley In Partial Fulfilment of the Requirements for the Award of the Degree DOCTOR OF PHILOSOPHY of the UNIVERSITY OF LONDON Christopher Ingold Laboratories Department of Chemistry University College London London WCIH OAJ September 1995 ProQuest Number: 10016731 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10016731 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Through doubting we come to questioning and through questioning we come to the truth. Peter Abelard, Paris, 1122 Abstract This thesis concerns an investigation into the generation and reactivity of organozinc carbenoids, from both a practical and mechanistic standpoint, using the reductive deoxygenation of carbonyl compounds with zinc and a silicon electrophile. The first introductory chapter is a review of organozinc carbenoids in synthesis. The second chapter opens with an overview of the development of the reductive deoxygenation of carbonyl compounds with zinc and a silicon electrophile since its inception in 1973. The factors influencing the generation of the zinc carbenoid are then investigated using a control reaction, and discussed. -

United States Patent Office
- 2,837,570 United States Patent Office Patented June 3, 1958 2 thesis of l-carvone from d-limonene glycol are repre sented as follows, along with the structural formulae of 2,837,570 l-carvone, d-limonene and d-limonene monoxide. For purposes of illustration, the intermediate substitution METHOD OF PREPARNG CARVONE 5 product formed in the dehydration and hydrolysis of the Seymour M. Linder, Eggertsville, and Frank P. Green 1-hydroxyhydrocarvone is represented as the semicar span, Buffalo, N.Y., assignors to Food Machinery and . bazone derivative of that compound. Chemical Corporation, San Jose, Calif. (His GH, CE No Drawing. Application March 21, 1956 -o COH Serial No. 572,789 10 6 Claims. (C. 260-587) Hic bH, C bH, NHMC NH/ 5 This invention relates to a method for preparing 1-car S n vone, and particularly to a method for preparing 1-car C /'sCH HC /o CH vone from d-limonene and certain d-limonene deriva : - d-limonene monoxide d-limonene glycol - tives. (H, CH 1-Carvone, the levoratory form of carvone, by reason COE O of its minty odor and flavor has found wide acceptance 20 / in the prepartion of cosmetics and comestibles. Typi HaC Yo-NNENH cally, it is used as a flavoring and perfuming ingredient for toothpaste, mouth wash, chewing gum and mint can dies. 1-Carvone is expensive, presently being obtained 25 from principally from spearmint oil in which it occurs / Sn naturally. HC CH The high cost combined with the usefulness of 1-car -carvone vone offers a strong incentive to chemists to devise syn thetic methods for preparing the material. -

Alternative Solvents in Carvone Hydrogenation
Catarina Isabel Cabral de Carvalho e Melo Mestrado Integrado em Engenharia Química e Bioquímica Alternative Solvents in Carvone Hydrogenation Dissertação para obtenção do Grau de Mestre em Engenharia Química e Bioquimica Orientadora: Dra Ewa Bogel-Łukasik Júri: Presidente: Prof. Doutor Manuel Nunes da Ponte Arguente: Doutor Rafał Marcin Bogel-Łukasik Vogal: Doutora Ewa Bogel-Łukasik Julho de 2011 Copyright Os direitos de cópia da dissertação intitulada de “Alternative Solvents in Carvone Hydrogenation” pertencem ao autor, à Faculdade de Ciências e Tecnologia e à Universidade Nova de Lisboa. A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualguer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distrilbuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. ii Acknowledgments The accomplishment of this thesis was only possible due to the contribution of several people, to whom I would like to thank. First I would like to express my gratitude to my supervisor Dr. Ewa Bogel Łukasik for the ‐ supervision and all her help, and to Professor Dr. Manuel Nunes da Ponte, head of Chemical Engineering Department where I have had the opportunity to work. I would like to acknowledge Professor Dr. Marco Silva for all the help provided in order to make get along with the Gas Chromatograph. -

Steroids from Carvone Promotor Prof
Steroids from Carvone Promotor Prof. dr. Ae. de Groot, Hoogleraar in de Bio-organische Chemie, Wageningen Universiteit Co-promotoren Prof. dr. M. B. Groen, Hoogleraar aan de Vrije Universiteit Amsterdam Dr. B. J. M. Jansen, Universitair Docent bij het Laboratorium voor Organische Chemie, Wageningen Universiteit Promotiecommissie Prof. dr. J. Wicha, Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland Prof. dr. H. Hiemstra, Universiteit van Amsterdam Dr. J.W. Scheeren, Radboud Universiteit Nijmegen Prof. dr. E. J. R. Sudhölter, Wageningen Universiteit Florence C. E. Sarabèr Steroids from Carvone Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. ir. L. Speelman, in het openbaar te verdedigen op dinsdag 10 mei 2005 des namiddags te half twee in de aula Sarabèr, Florence C. E. Steroids from Carvone Thesis Wageningen University –with references and summaries in English, French and Dutch ISBN 90-8504-181-3 Contents Chapter 1 1 Introduction Chapter 2 31 Domino Mukaiyama reactions to polycyclic systems Chapter 3 63 New approach towards C,D-trans fused steroid and D-homo steroid skeletons Chapter 4 85 A new and short synthesis of C,D-cis fused steroid and D-homosteroid skeletons Chapter 5 99 A second new and short synthesis of C,D-trans fused steroid skeletons Chapter 6 127 Synthesis of a chiral ring D precursor for the generation of enantiomerically pure steroid skeletons Chapter 7 145 1-Phenylthio-3-vinyl-3-cyclohexenol, a new reagent for bis-annelation of silyl enol ethers Chapter 8 165 The use of b-cyanoketones for the synthesis of functionalized polycyclic compounds Chapter 9 177 Discussion Appendix 187 List of used abbreviations 188 Summary 189 Samenvatting 195 Résumé 201 Dankwoord 207 Curriculum vitae 209 Wanneer jij je ogen opent zullen wij, opnieuw, op weg gaan tussen de uren en hun uitvindingen en slenterend tussen de verschijningen zullen wij de tijd en zijn vervoegingen bevestigen. -

The Quantitative Determination of Carvone in Essential Oils
T H E S I S on THE QUANTITATIVE DETERMINATION OF CARVONE IN ESSENTIAL OILS Submitted to the OREGON STATE AGRICULTURAL COLLEGE In partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE by Jesse Carl Kimmel May 1932 APPROV@: Redacted for Privacy esson of Phanmacy In Charge Redacted for Privacy Chafu'man of Conmlttee on Graduate Study ACKNOWLEDGMENTS To Professor Lewis c. Britt, under whose direction this work was done, I wish to express my sincere appreciation for the kind assistance and encouragement which he was ready at all times to give. I also wish to express my appreciation of the help and inspiration that has come to me through my associations with Doctor F. A. Gilfillan and Professor E. T. Stuhr. TABLE OF CONTENTS I. Introduction II. Previous Work III. Experimental Approach rv. Sulphite-sulphate Method v. Conclusions VI. Appendix VII. Bibliography I NTRODUCTION The study of a method for the determination of carvone in the official1 volatile oils was begun after attempting to determine. the carvone content of oil of caraway by the method outlined in the United States Pharmacopoeia. The U. s. P. method is not satisfactory as the neutralization of the sodium hydroxide, formed by the hydrolysis of carvone sodium sulphite, cannot be accomplished in the size flask prescribed in the of ficial monograph. Assay by the U. S. P. IX2 method is also unsatisfactory as the accuracy is not greater than one per cent. In this method one hundredth part of a cubic centimeter must be estimated and multiplied by ten in order to arrive at the volume of carvone present.