United States Patent Office
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mint and Mint Oil Profile New York State Integrated Pest Management Cornell Cooperative Extension Program
http://hdl.handle.net/1813/56133 Mint and Mint Oil Profile New York State Integrated Pest Management Cornell Cooperative Extension Program Mint and Mint Oil Profile Active Ingredient Eligible for Minimum Risk Pesticide Use Brian P. Baker and Jennifer A. Grant New York State Integrated Pest Management, Cornell University, Geneva NY Label Display Names: Cornmint, Cornmint oil, U.S. EPA PC Code: 128800 (Peppermint is also Spearmint, Spearmint oil cross referenced under the listing for mint and mint oil) Active Components: Carvone, Menthol, pulegone, limonene, linarin, pinene, piperitone, linalool, and CA DPR Chem Code: Not Found various other terpenoids, alcohols, esters, and hydrocarbons Other Names: Cornmint oil, spearmint oil, men- thol oil, spearmint terpenes CAS Registry #s: 8008-79-5 (Spearmint oil) Other Codes: 68917-18-0 (Cornmint oil) Cornmint oil—FEMA: 4219; EINECS: 290-058-5; HT: None (Cornmint and Spearmint) 3301.25 Spearmint oil—FEMA: 3032; EINECS: 283-656- HT: 3301.25; RTECS: WG7360000 Summary: Mint and mint oils are a class of active ingredients derived from selected members of the plant genus Mentha. Primary sources include cornmint, peppermint, and spearmint. Active substances contained in the plants and essential oils in this class include menthol, carvone, and various other ter- penoids. Peppermint and spearmint are commonly used food ingredients; cornmint is used as a food ingredient in some Asian cuisines and is a major source of food-grade menthol used as a flavoring agent. As a pesticide, mint and mint oils work primarily through non-toxic modes of action as repellents, but also have anti-microbial properties. Peppermint is covered in a separate profile. -

United States Patent Patented Dec
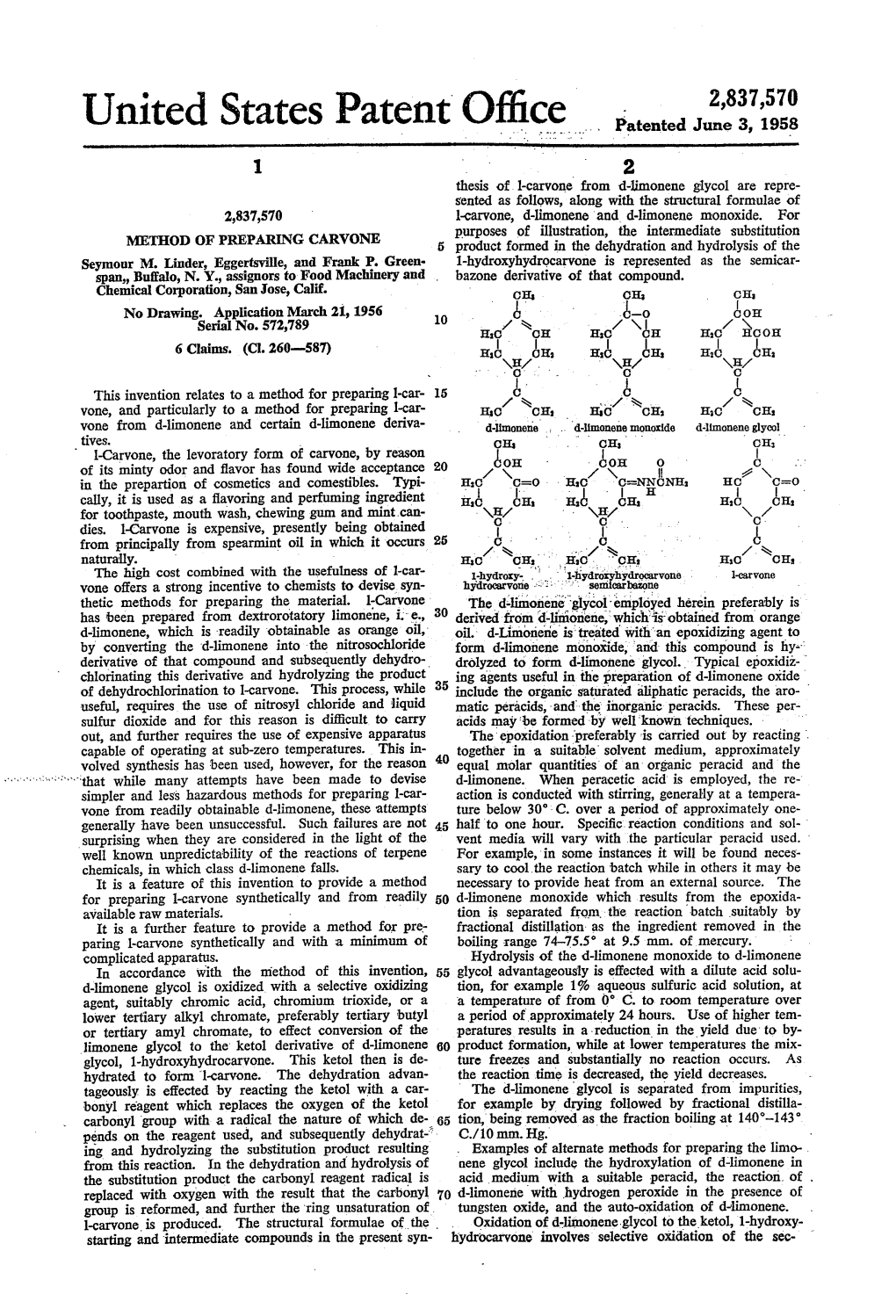
Mice 3,293,301 United States Patent Patented Dec. 20, 1966 1 2 tional acid per mole of carvoxime at the beginning of the 3,293,301 hydrolysis. Phosphoric acid is the preferred additional PREPARATION OF CARVONE acid. John M. Derfer, Jacksonville, Bernard J. Kane, Atlantic Also, by using an acceptor for the hydroxylamine it is Beach, and Donald G. Young, Jacksonville, Fla., as not necessary to remove the carvone during the hydrolysis signors to The Glidden Company, Cleveland, Ohio, a as in Reitsema, since there is an equilibrium between corporation of Ohio No Drawing. Filed Apr. 7, 1964, Ser. No. 358,051 carvoxime and its hydrolysis products, carvone and hy- ' 13 Claims. (Cl. 260-—-587) droxylamine, and the reaction can be carried to comple tion before any recovery of the carvone is required. The present invention relates to the preparation of We have found that the objects of the invention can be carvone. achieved when the reactions are carried ‘out in a suitable 'Carvone is an important constituent of many essential solvent such as a lower aliphatic alkanol. The preferred oils. About 65% of spearmint oil is l-carvone and d solvent is isopropyl alcohol. But any other solvent for carvone is the chief constituent of caraway and dill seed carvoxime and carvone which is water soluble, nonreac oils. 15 tive with the reacting materials, and which has a boiling The preparation of l-carvone from d-limonene has been point of at least about 75° C, or higher can be used. described by Bordenca et al., Ind. and Eng. -

Alternative Solvents in Carvone Hydrogenation
Catarina Isabel Cabral de Carvalho e Melo Mestrado Integrado em Engenharia Química e Bioquímica Alternative Solvents in Carvone Hydrogenation Dissertação para obtenção do Grau de Mestre em Engenharia Química e Bioquimica Orientadora: Dra Ewa Bogel-Łukasik Júri: Presidente: Prof. Doutor Manuel Nunes da Ponte Arguente: Doutor Rafał Marcin Bogel-Łukasik Vogal: Doutora Ewa Bogel-Łukasik Julho de 2011 Copyright Os direitos de cópia da dissertação intitulada de “Alternative Solvents in Carvone Hydrogenation” pertencem ao autor, à Faculdade de Ciências e Tecnologia e à Universidade Nova de Lisboa. A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualguer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distrilbuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. ii Acknowledgments The accomplishment of this thesis was only possible due to the contribution of several people, to whom I would like to thank. First I would like to express my gratitude to my supervisor Dr. Ewa Bogel Łukasik for the ‐ supervision and all her help, and to Professor Dr. Manuel Nunes da Ponte, head of Chemical Engineering Department where I have had the opportunity to work. I would like to acknowledge Professor Dr. Marco Silva for all the help provided in order to make get along with the Gas Chromatograph. -

Steroids from Carvone Promotor Prof
Steroids from Carvone Promotor Prof. dr. Ae. de Groot, Hoogleraar in de Bio-organische Chemie, Wageningen Universiteit Co-promotoren Prof. dr. M. B. Groen, Hoogleraar aan de Vrije Universiteit Amsterdam Dr. B. J. M. Jansen, Universitair Docent bij het Laboratorium voor Organische Chemie, Wageningen Universiteit Promotiecommissie Prof. dr. J. Wicha, Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland Prof. dr. H. Hiemstra, Universiteit van Amsterdam Dr. J.W. Scheeren, Radboud Universiteit Nijmegen Prof. dr. E. J. R. Sudhölter, Wageningen Universiteit Florence C. E. Sarabèr Steroids from Carvone Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. ir. L. Speelman, in het openbaar te verdedigen op dinsdag 10 mei 2005 des namiddags te half twee in de aula Sarabèr, Florence C. E. Steroids from Carvone Thesis Wageningen University –with references and summaries in English, French and Dutch ISBN 90-8504-181-3 Contents Chapter 1 1 Introduction Chapter 2 31 Domino Mukaiyama reactions to polycyclic systems Chapter 3 63 New approach towards C,D-trans fused steroid and D-homo steroid skeletons Chapter 4 85 A new and short synthesis of C,D-cis fused steroid and D-homosteroid skeletons Chapter 5 99 A second new and short synthesis of C,D-trans fused steroid skeletons Chapter 6 127 Synthesis of a chiral ring D precursor for the generation of enantiomerically pure steroid skeletons Chapter 7 145 1-Phenylthio-3-vinyl-3-cyclohexenol, a new reagent for bis-annelation of silyl enol ethers Chapter 8 165 The use of b-cyanoketones for the synthesis of functionalized polycyclic compounds Chapter 9 177 Discussion Appendix 187 List of used abbreviations 188 Summary 189 Samenvatting 195 Résumé 201 Dankwoord 207 Curriculum vitae 209 Wanneer jij je ogen opent zullen wij, opnieuw, op weg gaan tussen de uren en hun uitvindingen en slenterend tussen de verschijningen zullen wij de tijd en zijn vervoegingen bevestigen. -

The Quantitative Determination of Carvone in Essential Oils
T H E S I S on THE QUANTITATIVE DETERMINATION OF CARVONE IN ESSENTIAL OILS Submitted to the OREGON STATE AGRICULTURAL COLLEGE In partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE by Jesse Carl Kimmel May 1932 APPROV@: Redacted for Privacy esson of Phanmacy In Charge Redacted for Privacy Chafu'man of Conmlttee on Graduate Study ACKNOWLEDGMENTS To Professor Lewis c. Britt, under whose direction this work was done, I wish to express my sincere appreciation for the kind assistance and encouragement which he was ready at all times to give. I also wish to express my appreciation of the help and inspiration that has come to me through my associations with Doctor F. A. Gilfillan and Professor E. T. Stuhr. TABLE OF CONTENTS I. Introduction II. Previous Work III. Experimental Approach rv. Sulphite-sulphate Method v. Conclusions VI. Appendix VII. Bibliography I NTRODUCTION The study of a method for the determination of carvone in the official1 volatile oils was begun after attempting to determine. the carvone content of oil of caraway by the method outlined in the United States Pharmacopoeia. The U. s. P. method is not satisfactory as the neutralization of the sodium hydroxide, formed by the hydrolysis of carvone sodium sulphite, cannot be accomplished in the size flask prescribed in the of ficial monograph. Assay by the U. S. P. IX2 method is also unsatisfactory as the accuracy is not greater than one per cent. In this method one hundredth part of a cubic centimeter must be estimated and multiplied by ten in order to arrive at the volume of carvone present. -

Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint
molecules Article Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint Zhaohai Wu 1,2, Bie Tan 1,3, Yanhong Liu 1, James Dunn 4, Patricia Martorell Guerola 5, Marta Tortajada 5, Zhijun Cao 2 and Peng Ji 6,* 1 Department of Animal Science, University of California, Davis, CA 95616, USA 2 State Key Laboratory of Animal Nutrition, College of Animal Science and Technology, China Agricultural University, Beijing 100193, China 3 Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China 4 Applied Nutrition, ADM Animal Nutrition, Quincy, IL 62305, USA 5 Cell Biology Laboratory, ADM Biopolis, 46980 Valencia, Spain 6 Department of Nutrition, University of California, Davis, CA 95616, USA * Correspondence: [email protected]; Tel.: +1-530-752-6469; Fax: +1-530-752-0175 Received: 26 June 2019; Accepted: 30 July 2019; Published: 2 August 2019 Abstract: Natural antioxidants have drawn growing interest for use in animal feed and the food industry. In the current study, essential oils (EOs) obtained from hydrodistillation of three mentha species, including Mentha piperita (peppermint), Mentha spicata (native spearmint) and Mentha gracilis (Scotch spearmint), harvested in the Midwest region in the United States, were analyzed for their chemical composition using gas chromatography-mass spectrometry, and their antioxidant properties were assessed through chemical assays, in vitro cell culture modeling and in Caenorhabditis elegans (C. elegans). The activity of ferric iron reduction and free-radical scavenging capacity were assessed through chemical-based assays, including the reducing power assay, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, and Trolox equivalent antioxidant capacity assay (TEAC). -

Liquid-Phase Catalytic Oxidation of Limonene to Carvone Over ZIF-67(Co)
catalysts Article Liquid-Phase Catalytic Oxidation of Limonene to Carvone over ZIF-67(Co) Yizhou Li, Yepeng Yang, Daomei Chen, Zhifang Luo, Wei Wang, Yali Ao, Lin Zhang, Zhiying Yan * and Jiaqiang Wang * National Center for International Research on Photoelectric and Energy Materials, Yunnan Provincial Collaborative Innovation Center of Green Chemistry for Lignite Energy, Yunnan Province Engineering Research Center of Photocatalytic Treatment of Industrial Wastewater, The Universities’ Center for Photocatalytic Treatment of Pollutants in Yunnan Province, School of Chemical Sciences & Technology, Yunnan University, Kunming 650091, China; [email protected] (Y.L.); [email protected] (Y.Y.); [email protected] (D.C.); [email protected] (Z.L.); [email protected] (W.W.); [email protected] (Y.A.); [email protected] (L.Z.) * Correspondence: [email protected] (Z.Y.); [email protected] (J.W.); Tel.: +86-871-6503-1567 (Z.Y.); +86-871-6503-1567 (J.W.) Received: 15 March 2019; Accepted: 16 April 2019; Published: 21 April 2019 Abstract: Liquid-phase catalytic oxidation of limonene was carried out under mild conditions, and carvone was produced in the presence of ZIF-67(Co), cobalt based zeolitic imidazolate framework, as catalyst, using t-butyl hydroperoxide (t-BHP) as oxidant and benzene as solvent. As a heterogeneous catalyst, the zeolitic imidazolate framework ZIF-67(Co) exhibited reasonable substrate–product selectivity (55.4%) and conversion (29.8%). Finally, the X-ray diffraction patterns of the catalyst before and after proved that ZIF-67(Co) acted as a heterogeneous catalyst, and can be reused without losing its activity to a great extent. -

71 ~Hru) ~
INcaR~aRATED 71 ~HRU) ~ 3 /1rPf;<' ~ '", .. 1 Annual Report "IRIS Biological Experiments". Contract No. NAS5-11294 " Prepared for National Aeronaut~cs and Space Administration Goddard Space Flight Center Greenbelt, Maryland. 20771 ,.. ., , "" '"'b • " Prepared by BIOSPHERICS INC ORPORA TED 4928 Wyaconda Road ,Rockville, Maryland 20853 April 15, 1971 ,', -. Annual Report April 1970 - April 1971 . Contract No. NAS5-11294 BIOSPHERICS INCORPORATED IRIS BIOLOGICAL EXPERIMENTS TABLE OF CONTENTS ABSTRACT I. INTRODUCTION II. BIOLOGICAL STUDIES A. Inferences to be Made from Surface Phenomenon 1. Reflectance of Inorganic, Organic, and Biological Samples a. Inorganic Reststrahlen Effect b. Reflectance of Organic .and Biological Samples .. c. Effect of Combining Inorganic with Organic or Biological Material 2.' Materials or Phenomenon Yielding Black Body Spectral Responses 3. Developm.ent of a Strategy froIn Surface Data 4. Probable Distribution of Si02 on the Surface of Mars 5. Status o£ Obtaining Biological Inferences from Surface Phenomenon 14 Be Inferences to be Made from Atmospheric Analysis 16 .I,. Gases Involved in Terrestrial Metabolic Pathways 16 2. Gas Detection by the IRIS· 21 .3. .Gases Detected in the Earth's Atmosphere 23 4. Relationships BetweellAtmospheric Gasesand_.· Surface. Metabolic A~tivity . 23 .. - ,,<~. • i Annual Report April 1970 - April 1971 \ Contract No .. NAS5-ll294 I· \ BIOSPHERICS INCORPORATED I i 1. I TABLE OF CONTENTS i t (Continued) I'. Page a. Diurnal Fluctuations in Gas Concentrations 25' b. Isotopic Ratios of Elements 25 c. \ The Hitchcock/Lovelock Theory 26 d. Limitations of IRIS in Utilizing Theories to Make Biological Implications 27 5. Presence of Hydrocarbons and Related Derivatives in the Earth's Atmosphere 28 a. Co.nunents on'1IBiue H.aze" b. -

Primary Documents. 27. on the Catalytic Decomposition Of
Bull. Hist. Chem., VOLUME 40, Number 2 (2015) 69 PRIMARY DOCUMENTS 27. ON THE CATALYTIC DECOMPOSITION OF ALKYLIDENEHYDRAZINES. (SECOND PART) (ABRIDGED) N. Kizhner Zhurnal Russkago Fiziko-Khimicheskago Obshchestva, 1911, 43, 951-962. Translated by Vladislav Suntsov and David E. Lewis Supplemental Material In the previous article, a method for obtaining carvone (from the bisulfite compound): boiling point hydrocarbons based on the catalytic decomposition of 222.5° at 752 mm.; [a]D = –18.36°. alkylidenehydrazines of the type R:N–NH in the pres- 2 The compound of dihydrocarvone with hydrazine, ence of potassium hydroxide, was described. C10H16:NNH2, was obtained as follows: a solution of 17 In this direction, so far only alkylidenehydrazines g. of dihydrocarvone and 25 g. 50% hydrazine hydrate in originating from ketones (cyclic and bicyclic series) alcohol was boiled under reflux for 5 hours, after which have been studied. In terms of the generalization of the the alcohol and part of the hydrazine hydrate were dis- method, it was interesting to explore the decomposition tilled from an oil bath (bath temperature 140°). The base of alkylidenehydrazines of the aldehyde type, as well as was dried by warming with fused potash on a water bath. to extend it to hydrazine derivatives with the character The decomposition of the base when heated with of an unsaturated ketone. potassium hydroxide is very easy. The hydrogen [hydro- In this paper, the extension of our method to two carbon? —translators] was distilled with steam, washed series of unsaturated cyclic ketones, dihydrocarvone with 50% acetic acid, then with water, and dried with and carvenone, and the two unsaturated fatty aldehydes, calcium chloride. -

Technical Document for L-Carvone Also Referred to As a BRAD
BIOPESTICIDES REGISTRATION ACTION DOCUMENT l-Carvone U.S. Environmental Protection Agency Office of Pesticide Programs Biopesticides and Pollution Prevention Division (Last updated August 31, 2009) This document is for informational purposes only and is representative of the Agency’s justification in registering products containing this active ingredient. This is not a legal document. l-Carvone Page 2 of 16 Biopesticides Registration Action Document TABLE OF CONTENTS I. EXECUTIVE SUMMARY.................................................................................................... 5 II. ACTIVE INGREDIENT OVERVIEW .................................................................................. 6 III. REGULATORY BACKGROUND........................................................................................ 6 A. Food Clearances/Tolerances.................................................................................................. 6 IV. RISK ASSESSMENT............................................................................................................6 A. Active Ingredient Characterization........................................................................................ 6 B. Human Health Assessment .................................................................................................... 7 1. Toxicology .......................................................................................................................... 7 a. Acute Toxicity............................................................................................................... -

Methylene-P-Toluenesulfonamide (−)-Carvone Is Spearmint Oil, Whereas the (S)-(+)- a B Enantiomer Is a Constituent of Dill and Caraway Oils
Note 351 Synthesis and Analgesic-like Effect diterpene skeletons. Among the monoterpene starting − of (6R,4S)-p-Mentha-1,8-dien-6-yl- materials, probably the (+)- and ( )-forms of car- vone are the most versatile. The best source of (R)- methylene-p-toluenesulfonamide (−)-carvone is spearmint oil, whereas the (S)-(+)- a b enantiomer is a constituent of dill and caraway oils. Dami˜ao P. de Sousa , Franklin F. F. Nobrega ´ , The cost of (R)-(−)-carvone is usually much lower Reinaldo N. de Almeidab,andTimothyJ.Brocksomc than that of the (+)-isomer, but both enantiomers of a Laborat´orio de Qu´ımica de Produtos Naturais e Sint´eticos carvone have been used as chirons in the synthesis of Bioativos (LAPROBIO), Departamento de Fisiologia, Universidade Federal de Sergipe, diverse intermediates and natural compounds, princi- CEP 49100-000 S˜ao Crist´ov˜ao, Sergipe, Brazil pally terpenoids [1 – 4]. b Laborat´orio de Tecnologia Farmacˆeutica, Universidade The range of pharmacological activity that has been Federal da Para´ıba; Caixa Postal 5009, CEP 58051 – 970, recorded for terpenes is remarkably wide. They are Jo˜ao Pessoa, Para´ıba, Brazil c Laborat´orio de Qu´ımica Bio-Orgˆanica, Departamento de found to function as anticonvulsant, antinociceptive, Qu´ımica, Universidade Federal de S˜ao Carlos, sedative, and anxiolytic agents [5 – 11]. The range Caixa Postal 676, 13565-905 S˜ao Carlos, SP, Brazil of pharmacological effects of this family of natu- Reprint requests to Dr. Dami˜ao P. de Sousa. ral products is apparently due to a variety of ac- E-mail: damiao [email protected] tion mechanisms. -

Chapter I I I Transformations of (-) Carvone
chapter I I I transformations o f (- ) carvone 96 Michael addition in its original scope is the addition of an addend or donor (A) containing an o(-H utom in the system C=C-C-H to a carbon - carbon double bond that forms part of a conjugated system of the general formula in an acceptor (B), Rl R2 R4 He ^7 ^2 R4 Re R? ! I I ' I Base i i I j I 0 = C - C - H + C = C - C = 0-^0 = C-C-C-C-C=0 I R3 H5 H (A) (ii) The condensation takes place under the influence of alkaline reagents. By extension of original scope, Michael condensation has come to be understood to include addends and acceptors activated by groups other than carbonyl and carboxyl. Michael reaction has been widely used for the synthesis of a variety of organic compounds.’*' 2 AS a typical example the Robinson ring annelation, a key reaction for the synthesis of many terpenes and steroids, may be cited. ♦ For review see reference 1. (I) (II) ( III ) (IV) (V) HO HO. 0 . .0 0 (VI) ( VII ) ( VIII ) ( IX) (X) 0 .0 (XI) (XII) (XIII) NNHCONH. NNHCONH. NNHCONH. (XIII a) (XIII b) (XIV) NNHCgH3(N02); NNHCgH3(N02): .NNHCgH3(N 02); (XV) (XVa) (XVb) /) OH OH ? Y (XVI) (XVII) (XVIII) (XIX) (XX) OTs /k,>.>COOH i „«COOEf ( XXI ) (XXII) (XXIII) ( XXIV ) OH ( XXV ) (XXVI) (XXVII) (XXVIII) OTs (XXIX ) (XXX) (XXXI) (XXXII) EtOOC HOOCHpC EtOOC (XXXIII) ( XXXIV) (XXXV) (XXXVI) 97 The addition of a nucleophllic reagent to carvone (I) will furnish the enolate (Ci) or (Cg), which will then be transformed to (D^^) or (D2).