Characterization of Lower Limb Muscle Activation Patterns During Walking and Running with Intravoxel Incoherent Motion (IVIM) MR Perfusion T Imaging ⁎ Pia M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
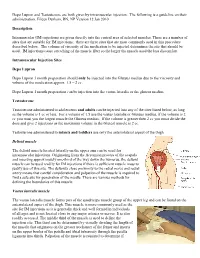
An Intramuscular Injection Is an Injection Given Directly Into The
Depo Lupron and Testosterone are both given by intramuscular injection. The following is a guideline on their administration. Eileen Durham, RN, NP Version 12 Jan 2010 Description Intramuscular (IM) injections are given directly into the central area of selected muscles. There are a number of sites that are suitable for IM injections; there are three sites that are most commonly used in this procedure described below. The volume of viscosity of the medication to be injected determines the site that should be used. IM injections cause stretching of the muscle fiber so the larger the muscle used the less discomfort. Intramuscular Injection Sites Depo Lupron Depo Lupron 3 month preparation should only be injected into the Gluteus medius due to the viscosity and volume of the medication approx. 1.5 – 2 cc. Depo Lupron 1 month preparation can be injection into the vastus lateralis or the gluteus medius. Testosterone Testosterone administered to adolescents and adults can be injected into any of the sites listed below, as long as the volume is 1 cc or less. For a volume of 1.5 use the vastus lateralis or Gluteus medius, if the volume is 2 cc you must you the largest muscle the Gluteus medius. If the volume is greater then 2 cc you must divide the dose and give 2 injections as the maximum volume in the Gluteal muscle is 2 cc. Testosterone administered to infants and toddlers use only the anteriolateral aspect of the thigh. Deltoid muscle The deltoid muscle located laterally on the upper arm can be used for intramuscular injections. -

Bacterial Meningitis and Multiple Abscess Formation in the Iliopsoas
Yamada et al. Renal Replacement Therapy (2018) 4:22 https://doi.org/10.1186/s41100-018-0163-x CASEREPORT Open Access Bacterial meningitis and multiple abscess formation in the iliopsoas, erector spinae, and vastus lateralis muscle in a maintenance hemodialysis patient treated with continuous epidural anesthesia for herpes zoster-related pain control: a case report and review of the literature Shunsuke Yamada1, Narihito Tatsumoto1, Noriko Nakamura1, Kosuke Masutani1, Toshiro Maeda2, Takanari Kitazono1 and Kazuhiko Tsuruya1,3* Abstract Background: Infection is the second leading cause of mortality in patients who undergo maintenance hemodialysis. In this population, impairment in both cellular and humoral immunity contributes to the increased incidence of infection and infection-related hospitalization. However, these artificial devices occasionally enhance the risk of deep organ infection including muscle abscess, frequently leading to disability and mortality in hemodialysis patients. Case presentation: A 54-year-old male undergoing maintenance hemodialysis was hospitalized because of the acute onset pain in the back and bilateral legs and declining consciousness which started at 7 days after the treatment of herpes zoster-related neuralgia with continuous epidural anesthesia. Physical examination revealed purulent discharge from the insertion site of the catheter. Serum biochemical tests showed increased inflammatory response and malnutrition. Magnetic resonance imaging revealed meningitis and multiple abscesses in the iliopsoas, erector spinae, gluteus medius, and vastus lateralis muscles, where conventional antibiotic treatment often fails to cure. Staphylococcus aureus was detected in the cerebrospinal fluid. Combination of intravenous antibiotics treatment and aggressive open surgical drainage of the muscle abscesses finally cured meningitis and multiple deep muscle abscesses in this patient. -

Applications of the Pedicled Vastus Lateralis Flap for Patients with Complicated Pressure Sores
Spinal Cord (1997) 35, 437 ± 442 1997 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/97 $12.00 Applications of the pedicled vastus lateralis ¯ap for patients with complicated pressure sores AB Schmidt1, G Fromberg1 and M-H Ruidisch2 1Abt. fuÈr Plastische-, Hand-, Kiefer- und rekonstruktive Mikrochirurgie, BG Unfallklinik Murnau; 2Abt. fuÈr RuÈckenmark- und WirbelsaÈulenverletztungen, BG Unfallklinik Murnau, Germany The vastus lateralis muscle- or musculocutaneous ¯ap is a well established tool in the surgery of pressure sores of the pelvic region. Its size, its constant large axial vascular pedicle originating from the lateral circum¯ex femoral artery, and its ability to carry quite a large skin island from the distal lateral region of the upper thigh makes this ¯ap a very versatile one in the management of dicult situations. The vastus lateralis ¯ap allows the simultaneous closure of defects in the trochanteric and sacral region, a technique which has not been described previously. A pedicled vastus lateralis ¯ap may be the only remaining local salvage procedure for defects due to obstruction of internal and external iliac arteries and aortobifemoral bypass surgery. This is another application which has not yet been described in the medical literature. The outcome of a series of 38 vastus lateralis ¯aps and the complications are shown. The follow-up period ranged from 3 months to 4 years. One ¯ap was lost. More complications were seen at the donor site than at the reconstructed defect. In patients who may be able to walk or stand at a later date, potential impairment of these functions has to be considered. -

The Role of and Relationship Between Hamstring and Quadriceps Muscle Myofascial Trigger Points in Patients with Patellofemoral Pain Syndrome
The role of and relationship between Hamstring and Quadriceps muscle myofascial trigger points in patients with patellofemoral pain syndrome. By Karen Louise Frandsen Smith A mini-dissertation submitted in partial compliance with the requirements for the Master’s Degree in Technology: Chiropractic at the Durban University of Technology I, Karen Louise Frandsen Smith, declare that this dissertation is representative of my own work in both conception and execution (except where acknowledgements indicate to the contrary). _______________________ ________________ Karen Louise Frandsen Smith Date Approved for Submission _______________________ ________________ Dr Brian Kruger (supervisor) Date M. Tech. Chiro., C.C.S.P. Dedication I dedicate this work to everyone who loves me and have supported me throughout these years of studying and all the difficult times. It is thanks to you that I have reached my dream. Dad, you would be so proud. i Acknowledgements Thank you to the DUT staff, patients, and supervisor, Dr Brian Kruger for making this happen. Thanks to my class mates for making the years fly by, and creating lifelong memories. This dissertation would not have been completed without supportive, generous and helpful people. Special thanks to you, Dr Danella Lubbe for motivating me and thank you so much Dr Charmaine Korporaal for “picking me up” and helping me finish. Endless gratitude goes to you Damon, Cherine, Mom and Viggo. You are / were all my anchors in the storm, and without your belief in me, I would be nowhere. ii Abstract Purpose: Patellofemoral Pain Syndrome is a common condition in all age groups, with a multi- factorial etiology. -

Chapter 10 the Knee Joint
The Knee Joint • Knee joint – largest joint in body Chapter 10 – very complex The Knee Joint – primarily a hinge joint Manual of Structural Kinesiology Modified for Prentice WE: Arnheim’s principles of athletic training , ed 12, New R.T. Floyd, EdD, ATC, CSCS York, 2006, McGraw-Hill; from Saladin, KS: Anatomy &physiology: the unity of forms and function , ed 2, New York, 2001, McGraw- Hill. © 2007 McGraw-Hill Higher Education. All rights reserved. 10-1 © 2007 McGraw-Hill Higher Education. All rights reserved. 10-2 Bones Bones • Enlarged femoral condyles articulate on • Fibula - lateral enlarged tibial condyles – serves as the attachment for • Medial & lateral tibial condyles (medial & knee joint lateral tibial plateaus) - receptacles for structures femoral condyles – does not articulate • Tibia – medial with femur or patella – bears most of weight – not part of knee joint Modified from Anthony CP, Kolthoff NJ: Textbook of anatomy and physiology , ed 9, St. Louis, 1975, Mosby. © 2007 McGraw-Hill Higher Education. All rights reserved. 10-3 © 2007 McGraw-Hill Higher Education. All rights reserved. 10-4 Bones Bones • Patella • Key bony landmarks – sesamoid (floating) bone – Superior & inferior patellar poles – imbedded in quadriceps – Tibial tuberosity & patellar tendon – Gerdy’s tubercle – serves similar to a pulley – Medial & lateral femoral in improving angle of condyles pull, resulting in greater – Upper anterior medial tibial mechanical advantage in surface – Head of fibula knee extension Modified from Anthony CP, Kolthoff NJ: Textbook of anatomy and physiology , ed 9, St. Louis, 1975, Mosby. © 2007 McGraw-Hill Higher Education. All rights reserved. 10-5 © 2007 McGraw-Hill Higher Education. All rights reserved. -

Anatomic Study of the Portions Long and Oblique of the Vastus Lateralis and Vastus Medialis Muscles: Review Article
Review article Anatomic study of the portions long and oblique of the vastus lateralis and vastus medialis muscles: review article Vieira, EPL.* Departamento de Educação Física, Universidade Salgado de Oliveira – UNIVERSO, São Gonçalo, RJ, Brazil *E-mail: [email protected] Abstract Although not mentioned directly in the classical anatomical literature, the vastus lateralis and vastus medialis, which make up the quadriceps femoris muscle, show variations in their anatomical structures due to the presence of long and oblique portions receiving the designation of vastus lateralis longus, vastus lateralis obliques to the vastus lateralis and vastus medialis oblique and vastus long, for the vastus medialis. The aim of this paper is to review the scientific literature regarding the presence of long and oblique portions of the broad medial and lateral portions recognize these as integral parts, anatomically, the quadriceps femoris. To this end, we used published articles in magazines and journals, located through Medline, and Lilacs Excerpa Medica, and the Portal Capes, with the key words: quadriceps, vastus medialis, vastus lateralis, vastus medialis longus, vastus medialis oblique , vastus lateralis longus and vastus lateralis oblique. Used to, still, a master’s thesis, located at Portal Capes, plus textbooks and atlases of anatomy. Among the 27 surveyed, only two do not recognize these portions as independent structures, considering the differences in fiber orientation. Of the 18 studied anatomy books, no mention such parts. However, eight anatomy books describe differences in trajectory of fiber insertions of the vastus lateralis and vastus medialis. Before this study it was concluded that these portions have not only morphological differences but also in other respects proved through scientific studies being published in some of them considered independent muscle suggesting inclusion of muscles in Anatomical Nomina. -

Motor Nerve to the Vastus Lateralis
ORIGINAL ARTICLE Motor Nerve to the Vastus Lateralis Peter C. Revenaugh, MD; P. Daniel Knott, MD; Jennifer M. McBride, PhD; Michael A. Fritz, MD Objective: To further delineate the anatomy of the mo- There were 2 larger caliber branches (Ͼ2 mm in diam- tor nerve to the vastus lateralis (MNVL) in the context eter) supplying the proximal and distal muscle. The nerve of its use as a possible interpositional nerve graft in fa- branches are variable in their relation to the vascular cial nerve rehabilitation. pedicle and perforating vessels of the descending branch of the lateral circumflex femoral artery. Methods: Twelve fresh human cadaveric thighs were dissected to investigate the anatomic location and branch- Conclusion: The nerve to the vastus lateralis is a read- ing pattern of the MNVL muscle. ily available, redundant motor nerve suitable for facial nerve cable grafting. Results: There were 3 to 6 primary nerve branches (mean, 4.4) supplying the vastus lateralis. The mean primary Arch Facial Plast Surg. 2012;14(5):365-368. branch length was 93.8 mm (range, 51-196 mm), and each Published online April 16, 2012. primary branch had a mean of 2.3 subsequent branches. doi:10.1001/archfacial.2012.195 ACIAL NERVE PARALYSIS IS A describe the anatomic distribution of the potentially devastating cra- MNVL in cadaveric dissections and dis- nial nerve injury with both cuss the novel use of the nerve as a cable functional and psychologi- graft in facial nerve grafting. cal impact. Neural compro- Fmise following tumor resection or in cases METHODS of traumatic and iatrogenic injury can be treated in several ways. -

A Newly Discovered Muscle: the Tensor of the Vastus Intermedius
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Published in &OLQLFDO$QDWRP\ ± which should be cited to refer to this work. provided by RERO DOC Digital Library A Newly Discovered Muscle: The Tensor of the Vastus Intermedius 1 2 2 3 4 K. GROB, * T. ACKLAND, M.S. KUSTER, M. MANESTAR, AND L. FILGUEIRA 1Department of Orthopaedic Surgery, Kantonsspital St. Gallen, St. Gallen, Switzerland 2The University of Western Australia, Perth, Australia 3Department of Anatomy, University of Zurich-Irchel,€ Zurich,€ Switzerland 4Department of Anatomy, University of Fribourg, Fribourg, Switzerland The quadriceps femoris is traditionally described as a muscle group com- posed of the rectus femoris and the three vasti. However, clinical experience and investigations of anatomical specimens are not consistent with the text- book description. We have found a second tensor-like muscle between the vastus lateralis (VL) and the vastus intermedius (VI), hereafter named the tensor VI (TVI). The aim of this study was to clarify whether this intervening muscle was a variation of the VL or the VI, or a separate head of the exten- sor apparatus. Twenty-six cadaveric lower limbs were investigated. The architecture of the quadriceps femoris was examined with special attention to innervation and vascularization patterns. All muscle components were traced from origin to insertion and their affiliations were determined. A TVI was found in all dissections. It was supplied by independent muscular and vascular branches of the femoral nerve and lateral circumflex femoral artery. Further distally, the TVI combined with an aponeurosis merging separately into the quadriceps tendon and inserting on the medial aspect of the patella. -

Effects of Rest Position on Morphology of the Vastus Lateralis and Its Relationship with Lower-Body Strength and Power
Journal of Functional Morphology and Kinesiology Article Effects of Rest Position on Morphology of the Vastus Lateralis and Its Relationship with Lower-Body Strength and Power Alyssa N. Varanoske 1, Nicholas A. Coker 1, Bri-Ana D.I. Johnson 1, Tal Belity 1, Gerald T. Mangine 2 , Jeffrey R. Stout 1, David H. Fukuda 1 and Adam J. Wells 1,* 1 Institute of Exercise Physiology and Rehabilitation Science, Division of Kinesiology, University of Central Florida, 12494 University Blvd., Orlando, FL 32816, USA 2 Department of Exercise Science and Sport Management, Kennesaw State University, Kennesaw, GA 30144, USA * Correspondence: [email protected]; Tel.: +1-407-823-3906 Received: 1 August 2019; Accepted: 20 August 2019; Published: 3 September 2019 Abstract: Ultrasonography of the lower body typically encompasses supine rest due to fluid shifts affecting tissue size and composition. However, vastus lateralis (VL) examination is completed in the lateral recumbent position, and this positional change may influence morphology and its ability to predict function. This study aimed to examine the effect of position on VL morphology and its relationship with lower-body performance. Cross-sectional area (CSA), muscle thickness (MT), pennation angle (PA), echo intensity (UnCorEI), subcutaneous adipose tissue thickness (SFT), and echo intensity corrected for SFT (CorEI) were assessed in 31 resistance-trained males (23.0 2.1 yrs; ± 1.79 0.08 m; 87.4 11.7 kg) immediately after transitioning from standing to supine (IP), after 15 min ± ± of standing (ST), and after 15 min of rest in three recumbent positions: supine (SUP), dominant lateral recumbent (DLR), non-dominant lateral recumbent (NDLR). -

Mononeuropathy of a Distal Branch of the Femoral Nerve in a Body Building Champion
Journal of Neurology, Neurosurgery, and Psychiatry 1997;63:669–671 669 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.63.5.669 on 1 November 1997. Downloaded from SHORT REPORT Mononeuropathy of a distal branch of the femoral nerve in a body building champion L Padua, E D’Aloya, M LoMonaco, R Padua, B Gregori, P Tonali Abstract Keywords: femoral nerve; mononeuropathy; body A unique case of a body building cham- building; anatomical study pion with localised atrophy of the distal portion of the vastus lateralis muscle is reported; neurophysiological evaluation Femoral mononeuropathy is uncommon.1–4 suggests a selective lesion of a distal Usually the lesion is located in the proximal branch of the vastus lateralis nerve (a tract, involving all muscles innervated by the motor branch of the femoral nerve). A femoral nerve. One case with an idiopathic iso- necroscopic study in four cases was per- lated femoral mononeuropathy aVecting the formed to better clarify the site and whole vastus lateralis has been recently mechanism of nerve lesion. The data sug- described,5 but no cases with partial atrophy of gest that stretching and compression of the vastus lateralis have been reported. the nerve has probably occurred during We report an atypical case of femoral strenous exercise. mononeuropathy. To identify the putative site copyright. of lesion an anatomical necroscopic study was (J Neurol Neurosurg Psychiatry 1997;63:669–671) performed in four cases. http://jnnp.bmj.com/ Istituto di Neurologia L Padua M LoMonaco B Gregori P Tonali Istituto di Medicina Legale E D’Aloya on September 30, 2021 by guest. -
Intramuscular Injections Policy
Fluid and Medication Management Intramuscular Injection protocol Intramuscular Injection Contents Purpose .......................................................................................................................................................... 1 Scope ............................................................................................................................................................. 1 Definitions ..................................................................................................................................................... 1 Associated documents .................................................................................................................................. 2 Statements .................................................................................................................................................... 2 Child administration ...................................................................................................................................... 2 Appropriate needle sizes (guide) .............................................................................................................. 3 Lippincott procedures and references (continue for figure diagrams not covered in Lippincott) ............... 4 Vastus Laterailis injection site information and figures ................................................................................ 4 Information .............................................................................................................................................. -
Comparison of Hamstrings and Quadriceps Muscle Activation in Male and Female Professional Soccer Players
applied sciences Article Comparison of Hamstrings and Quadriceps Muscle Activation in Male and Female Professional Soccer Players Gonzalo Torres † , Estrella Armada-Cortés † , Javier Rueda, Alejandro F. San Juan ‡ and Enrique Navarro *,‡ Health and Human Performance Department, Sport Biomechanics Laboratory, Faculty of Physical Activity and Sports Sciences-INEF, Universidad Politécnica de Madrid, 28040 Madrid, Spain; [email protected] (G.T.); [email protected] (E.A.-C.); [email protected] (J.R.); [email protected] (A.F.S.J.) * Correspondence: [email protected] † First two authors contributed equally to this work. ‡ E. Navarro and A. F. San Juan share senior authorship. Featured Application: Surface electromyography is a valid and useful tool to assess quadriceps and hamstring muscle activation in professional male and female soccer teams. This methodol- ogy can be used in hamstring and knee injury prevention and rehabilitation programs. Abstract: (1) Background: this study aimed to determine if there are differences in quadriceps and hamstring muscle activation in professional male and female soccer players. (2) Methods: muscle activation was recorded by surface electromyography in 27 professional soccer players (19 male and 8 female). The players performed the Bulgarian squat and lunge exercises. Vastus medialis, vastus lateralis, rectus femoris, semitendinosus, and biceps femoris were the muscles analyzed. (3) Results: The statistical analysis of the hamstring:quadriceps ratio showed no significant differences (p > 0.05). Significant differences were found in the vastus medialis:vastus lateralis ratio for both the lunge exercise (t20 = 3.35; p = 0.001; d = 1.42) and the Bulgarian squat (t23 = 4.15; p < 0.001; d = 1.76).