Standard for the Administration of Immunizations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
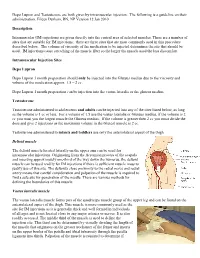
An Intramuscular Injection Is an Injection Given Directly Into The
Depo Lupron and Testosterone are both given by intramuscular injection. The following is a guideline on their administration. Eileen Durham, RN, NP Version 12 Jan 2010 Description Intramuscular (IM) injections are given directly into the central area of selected muscles. There are a number of sites that are suitable for IM injections; there are three sites that are most commonly used in this procedure described below. The volume of viscosity of the medication to be injected determines the site that should be used. IM injections cause stretching of the muscle fiber so the larger the muscle used the less discomfort. Intramuscular Injection Sites Depo Lupron Depo Lupron 3 month preparation should only be injected into the Gluteus medius due to the viscosity and volume of the medication approx. 1.5 – 2 cc. Depo Lupron 1 month preparation can be injection into the vastus lateralis or the gluteus medius. Testosterone Testosterone administered to adolescents and adults can be injected into any of the sites listed below, as long as the volume is 1 cc or less. For a volume of 1.5 use the vastus lateralis or Gluteus medius, if the volume is 2 cc you must you the largest muscle the Gluteus medius. If the volume is greater then 2 cc you must divide the dose and give 2 injections as the maximum volume in the Gluteal muscle is 2 cc. Testosterone administered to infants and toddlers use only the anteriolateral aspect of the thigh. Deltoid muscle The deltoid muscle located laterally on the upper arm can be used for intramuscular injections. -

Study Protocol and Statistical Analysis Plan
The University of Texas Southwestern Medical Center at Dallas Institutional Review Board PROJECT SUMMARY Study Title: Ultrasound-guided fascia iliaca compartment block versus periarticular infiltration for pain management after total hip arthroplasty: a randomized controlled trial Principal Investigator: Irina Gasanova, MD Sponsor/Funding Source: Department of Anesthesiology and Pain Management, UT Southwestern Medical School IRB Number: STU 122015-022 NCT Number: NCT02658240 Date of Document: 01 April 2016 Page 1 of 7 Purpose: In this randomized, controlled, observer-blinded study we plan to evaluate ultrasound-guided fascia iliaca compartment block with ropivacaine and periarticular infiltration with ropivacaine for postoperative pain management after total hip arthroplasty (THA). Background: Despite substantial advances in our understanding of the pathophysiology of pain and availability of newer analgesic techniques, postoperative pain is not always effectively treated (1). Optimal pain management technique balances pain relief with concerns about safety and adverse effects associated with analgesic techniques. Currently, postoperative pain is commonly treated with systemic opioids, which are associated with numerous adverse effects including nausea and vomiting, dizziness, drowsiness, pruritus, urinary retention, and respiratory depression (2). Use of regional and local anesthesia has been shown to reduce opioid requirements and opioid-related side effects. Therefore, their use has been emphasized (3, 4, 5, 6). Fascia Iliaca compartment block (FICB) is a field block that blocks the nerves from the lumbar plexus supplying the thigh (i.e., lateral femoral cutaneous femoral and obturator nerves). The obturator nerve is sometimes involved in the FICB but probably plays little role in postoperative pain relief for most surgeries of the hip and proximal femur. -

Intravenous Therapy Procedure Manual
INTRAVENOUS THERAPY PROCEDURE MANUAL - 1 - LETTER OF ACCEPTANCE __________________________________________ hereby approves (Facility) the attached Reference Manual as of _____________________. (Date) The Intravenous Therapy Procedure Manual will be reviewed at least annually or more often when deemed appropriate. Revisions will be reviewed as they occur. Current copies of the Intravenous Therapy Procedure Manual shall be maintained at each appropriate nursing station. I have reviewed this manual and agree to its approval. __________________________ (Administrator) __________________________ (Director of Nursing) __________________________ (Medical Director) - 2 - TABLE OF CONTENTS TABLE OF CONTENTS INTRODUCTION A. Purpose 1 B. Local Standard of Practice 1 RESPONSIBILITIES A. Responsibilities: M Chest Pharmacy 1 B. Responsibilities: Administrator 1 C. Responsibilities: Director of Nursing Services (DON/DNS) 1 D. Skills Validation 2 AMENDMENTS GUIDELINES A. Resident Candidacy for IV Therapy 1 B. Excluded IV Medications and Therapies 1 C. Processing the IV Order 1 D. IV Solutions/Medications: Storage 2 E. IV Solutions/Medications: Handling 3 F. IV Solutions and Supplies: Destroying and Returning 4 G. IV Tubing 5 H. Peripheral IV Catheters and Needles 6 I. Central Venous Devices 7 J. Documentation and Monitoring 8 K. IV Medication Administration Times 9 L. Emergency IV Supplies 10 I TABLE OF CONTENTS PROTOCOLS A. IV Antibiotic 1 1. Purpose 2. Guidelines 3. Nursing Responsibilities B. IV Push 2 1. Purpose 2. Guidelines C. Anaphylaxis Allergic Reaction 4 1. Purpose 2. Guidelines 3. Nursing Responsibilities and Interventions 4. Signs and Symptoms of Anaphylaxis 5. Drugs Used to Treat Anaphylaxis 6. Physician Protocol PRACTICE GUIDELINES A. Purpose 1 B. Personnel 1 C. Competencies 1 D. -

Sodium Chloride Injection USP 0.9% Prefilled Syringes, for Intravascular Use Only ------WARNINGS and PRECAUTIONS ------Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION --------------------- DOSAGE FORMS AND STRENGTHS --------------------- These highlights do not include all the information needed to use • Supplied as a clear, colorless, odorless, sterile solution of Sodium Sodium Chloride Injection USP 0.9% safely and effectively. See Chloride 0.9% for intravenous administration (3) full prescribing information for Sodium Chloride Injection USP • Supplied in 50 mL and 125 mL prefilled syringes (3) 0.9%. ------------------------------- CONTRAINDICATIONS ------------------------------- • None (4) Sodium Chloride Injection USP 0.9% Prefilled Syringes, For Intravascular Use Only ----------------------- WARNINGS AND PRECAUTIONS ----------------------- Initial U.S. Approval: 2006 • Remove all air from the syringe and associated tubing prior to injection. (5.1) ----------------------------INDICATIONS AND USAGE ---------------------------- • For use in flushing compatible contrast agents through Liebel- • May cause fluid overload in patients with congestive heart failure, Flarsheim intravenous administration sets into indwelling severe renal insufficiency, and in clinical states with edema, sodium retention, or hypernatremia. (5.3) intravascular access devices (1) • Establish intravascular catheter patency prior to administration. ----------------------- DOSAGE AND ADMINISTRATION ----------------------- (5.4) • For single patient use only. (2) • Determine the volume of flush based on the imaging procedure, ------------------------------ ADVERSE REACTIONS ------------------------------ -

Bacterial Meningitis and Multiple Abscess Formation in the Iliopsoas
Yamada et al. Renal Replacement Therapy (2018) 4:22 https://doi.org/10.1186/s41100-018-0163-x CASEREPORT Open Access Bacterial meningitis and multiple abscess formation in the iliopsoas, erector spinae, and vastus lateralis muscle in a maintenance hemodialysis patient treated with continuous epidural anesthesia for herpes zoster-related pain control: a case report and review of the literature Shunsuke Yamada1, Narihito Tatsumoto1, Noriko Nakamura1, Kosuke Masutani1, Toshiro Maeda2, Takanari Kitazono1 and Kazuhiko Tsuruya1,3* Abstract Background: Infection is the second leading cause of mortality in patients who undergo maintenance hemodialysis. In this population, impairment in both cellular and humoral immunity contributes to the increased incidence of infection and infection-related hospitalization. However, these artificial devices occasionally enhance the risk of deep organ infection including muscle abscess, frequently leading to disability and mortality in hemodialysis patients. Case presentation: A 54-year-old male undergoing maintenance hemodialysis was hospitalized because of the acute onset pain in the back and bilateral legs and declining consciousness which started at 7 days after the treatment of herpes zoster-related neuralgia with continuous epidural anesthesia. Physical examination revealed purulent discharge from the insertion site of the catheter. Serum biochemical tests showed increased inflammatory response and malnutrition. Magnetic resonance imaging revealed meningitis and multiple abscesses in the iliopsoas, erector spinae, gluteus medius, and vastus lateralis muscles, where conventional antibiotic treatment often fails to cure. Staphylococcus aureus was detected in the cerebrospinal fluid. Combination of intravenous antibiotics treatment and aggressive open surgical drainage of the muscle abscesses finally cured meningitis and multiple deep muscle abscesses in this patient. -

Applications of the Pedicled Vastus Lateralis Flap for Patients with Complicated Pressure Sores
Spinal Cord (1997) 35, 437 ± 442 1997 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/97 $12.00 Applications of the pedicled vastus lateralis ¯ap for patients with complicated pressure sores AB Schmidt1, G Fromberg1 and M-H Ruidisch2 1Abt. fuÈr Plastische-, Hand-, Kiefer- und rekonstruktive Mikrochirurgie, BG Unfallklinik Murnau; 2Abt. fuÈr RuÈckenmark- und WirbelsaÈulenverletztungen, BG Unfallklinik Murnau, Germany The vastus lateralis muscle- or musculocutaneous ¯ap is a well established tool in the surgery of pressure sores of the pelvic region. Its size, its constant large axial vascular pedicle originating from the lateral circum¯ex femoral artery, and its ability to carry quite a large skin island from the distal lateral region of the upper thigh makes this ¯ap a very versatile one in the management of dicult situations. The vastus lateralis ¯ap allows the simultaneous closure of defects in the trochanteric and sacral region, a technique which has not been described previously. A pedicled vastus lateralis ¯ap may be the only remaining local salvage procedure for defects due to obstruction of internal and external iliac arteries and aortobifemoral bypass surgery. This is another application which has not yet been described in the medical literature. The outcome of a series of 38 vastus lateralis ¯aps and the complications are shown. The follow-up period ranged from 3 months to 4 years. One ¯ap was lost. More complications were seen at the donor site than at the reconstructed defect. In patients who may be able to walk or stand at a later date, potential impairment of these functions has to be considered. -

An Overview On: Sublingual Route for Systemic Drug Delivery
International Journal of Research in Pharmaceutical and Biomedical Sciences ISSN: 2229-3701 __________________________________________Review Article An Overview on: Sublingual Route for Systemic Drug Delivery K. Patel Nibha1 and SS. Pancholi2* 1Department of Pharmaceutics, BITS Institute of Pharmacy, Gujarat Technological university, Varnama, Vadodara, Gujarat, India 2BITS Institute of Pharmacy, Gujarat Technological University, Varnama, Vadodara, Gujarat, India. __________________________________________________________________________________ ABSTRACT Oral mucosal drug delivery is an alternative and promising method of systemic drug delivery which offers several advantages. Sublingual literally meaning is ''under the tongue'', administrating substance via mouth in such a way that the substance is rapidly absorbed via blood vessels under tongue. Sublingual route offers advantages such as bypasses hepatic first pass metabolic process which gives better bioavailability, rapid onset of action, patient compliance , self-medicated. Dysphagia (difficulty in swallowing) is common among in all ages of people and more in pediatric, geriatric, psychiatric patients. In terms of permeability, sublingual area of oral cavity is more permeable than buccal area which is in turn is more permeable than palatal area. Different techniques are used to formulate the sublingual dosage forms. Sublingual drug administration is applied in field of cardiovascular drugs, steroids, enzymes and some barbiturates. This review highlights advantages, disadvantages, different sublingual formulation such as tablets and films, evaluation. Key Words: Sublingual delivery, techniques, improved bioavailability, evaluation. INTRODUCTION and direct access to systemic circulation, the oral Drugs have been applied to the mucosa for topical mucosal route is suitable for drugs, which are application for many years. However, recently susceptible to acid hydrolysis in the stomach or there has been interest in exploiting the oral cavity which are extensively metabolized in the liver. -

Vaccine Administration Joellen Wolicki, BSN, RN and Elaine Miller, RN, BSN, MPH
Vaccine Administration JoEllen Wolicki, BSN, RN and Elaine Miller, RN, BSN, MPH This chapter summarizes best practices related to vaccine administration, a key factor in ensuring vaccination is as safe NOTES and effective as possible. Administration involves a series of actions: assessing patient vaccination status and determining needed vaccines, screening for contraindications and precautions, educating patients, preparing and administering vaccines properly, and documenting the vaccines administered. Professional standards for medication administration, manufacturer instructions, and organizational policies and procedures should always be followed when applicable. 6 Staff Training and Education Policies should be in place to validate health care professionals’ knowledge of, and skills in, vaccine administration. All health care professionals should receive comprehensive, competency- based training before administering vaccines. Training, including an observation component, should be integrated into health care professionals’ education programs including orientation for new staff and annual continuing education requirements for all staff. In addition, health care professionals should receive educational updates as needed, such as when vaccine administration recommendations are updated or when new vaccines are added to the facility’s inventory. Training should also be offered to temporary staff who may be filling in on days when the facility is short-staffed or helping during peak periods of vaccine administration such as influenza season. Once initial training has been completed, accountability checks should be in place to ensure staff follow all vaccine administration policies and procedures. Before Administering Vaccine Health care professionals should be knowledgeable about appropriate techniques to prepare and care for patients when administering vaccines. Assess for Needed Vaccines The patient’s immunization status should be reviewed at every health care visit. -

Chapter 1 Controlling Drug Delivery
chapter 1 Controlling drug delivery Overview In this chapter we will: & differentiate drug delivery systems according to their physical state & differentiate drug delivery systems according to their route of administration & differentiate drug delivery systems according to their type of drug release & discuss drug transport across epithelial barriers. Introduction KeyPoints & Continued developments in Pharmacotherapy can be defined as the treatment chemistry, molecular biology and prevention of illness and disease by means of and genomics support the drugs of chemical or biological origin. It ranks discovery and developments among the most important methods of medical of new drugs and new drug treatment, together with surgery, physical targets. & treatment, radiation and psychotherapy. There The drug delivery system are many success stories concerning the use of employed can control the pharmacological action of a drugs and vaccines in the treatment, prevention drug, influencing its and in some cases even eradication of diseases pharmacokinetic and (e.g. smallpox, which is currently the only subsequent therapeutic human infectious disease completely profile. eradicated). Although it is almost impossible to estimate the exact extent of the impact of pharmacotherapy on human health, there can be no doubt that pharmacotherapy, together with improved sanitation, better diet and better housing, has improved people’s health, life expectancy and quality of life. Tip Unprecedented developments in genomics Combinatorial chemistry is a way to and molecular biology today offer a plethora of build a variety of structurally related new drug targets. The use of modern chemical drug compounds rapidly and synthetic methods (such as combinatorial systematically. These are assembled chemistry) enables the syntheses of a large from a range of molecular entities number of new drug candidates in shorter times which are put together in different ‘ ’ than ever before. -

Aerogen Nebulizer)
Page 1 of Date of Original: 11/15/18 Date of Review: Date of Revision: 9/4/19 Respiratory Therapy POLICY & PROCEDURE MANUAL RIBAVIRIN ADMINISTRATION (Aerogen nebulizer) PURPOSE: 1. Standardize the administration and delivery of ribavirin by aerosol 2. To protect the patient and caregivers by standardizing precautions for ribavirin delivery POLICY: The patient room must be set up according to St Jude’s Environmental Health and Safety policy prior to the administration of Ribavirin. Room preparation is to be done by the nursing staff. *See procedure section below https://home.stjude.org/ehs/Pages/ribavirin-precautions.aspx • Negative pressure room is mandatory for Ribavirin administration. • Preparation of the room is the responsibility of the nursing staff. • Ribavirin will be delivered from pharmacy in primed Aerogen syringe set appropriate for use on the MedFusion 3500 syringe pump. • The syringe pumps will be used, maintained, and stored by the respiratory therapy department. • The PPE cart is the shared responsibility of the respiratory therapist who administers the first dose of ribavirin and the nursing coordinator for that area. • Proper sign including contact precautions and STOP will be shared responsibility of the nursing and respiratory care. • All instances of spilled or leaked ribavirin must be documented in the event reporting system. • Ribavirin may be delivered to a patient during mechanical ventilation in a very specific set of circumstances. The final decision on the use of ribavirin is at the discretion of the ICU physician on service. EQUIPMENT: • Negative pressure room • DeMistifier 2000 HEPA filtration system. • DeMistifier negative pressure bed enclosure with canopy. • MedFusion 3500 Syringe pump • Flowmeter • Aerogen vibrating mesh nebulizer • Delivery Supplies (O2 tubing, O2 reducer, large bore tubing, T adaptor, and aerosol face mask) • Prescribed medication • STOP sign for the door during drug administration PPE • N95 or Positive Air Pressure mask required • Chemo resistant Gown • Double Gloves • Shoe covers PROCEDURE: 1. -

The Role of and Relationship Between Hamstring and Quadriceps Muscle Myofascial Trigger Points in Patients with Patellofemoral Pain Syndrome
The role of and relationship between Hamstring and Quadriceps muscle myofascial trigger points in patients with patellofemoral pain syndrome. By Karen Louise Frandsen Smith A mini-dissertation submitted in partial compliance with the requirements for the Master’s Degree in Technology: Chiropractic at the Durban University of Technology I, Karen Louise Frandsen Smith, declare that this dissertation is representative of my own work in both conception and execution (except where acknowledgements indicate to the contrary). _______________________ ________________ Karen Louise Frandsen Smith Date Approved for Submission _______________________ ________________ Dr Brian Kruger (supervisor) Date M. Tech. Chiro., C.C.S.P. Dedication I dedicate this work to everyone who loves me and have supported me throughout these years of studying and all the difficult times. It is thanks to you that I have reached my dream. Dad, you would be so proud. i Acknowledgements Thank you to the DUT staff, patients, and supervisor, Dr Brian Kruger for making this happen. Thanks to my class mates for making the years fly by, and creating lifelong memories. This dissertation would not have been completed without supportive, generous and helpful people. Special thanks to you, Dr Danella Lubbe for motivating me and thank you so much Dr Charmaine Korporaal for “picking me up” and helping me finish. Endless gratitude goes to you Damon, Cherine, Mom and Viggo. You are / were all my anchors in the storm, and without your belief in me, I would be nowhere. ii Abstract Purpose: Patellofemoral Pain Syndrome is a common condition in all age groups, with a multi- factorial etiology. -

IFU for Bioglue Surgical Adhesive (Outside USA)
Instructions for Use Syringe BioGlue® Adhésif BioGlue® Collante Chirurgico Adhesivo Quirúrgico Chirurgical Mode Chirurgischer Klebstoff BioGlue® Istruzioni d’uso BioGlue® Instrucciones d’emploi Seringue Gebrauchsanwei sung Siringa de Uso Jeringuilla Spritze BioGlue® Adesivo BioGlue® Kirurgisk BioGlue® Kirurgisk Lim BioGlue® Vävnadslim Cirúrgico Instruções de Klæbemiddel Bruksanvisning Sprøyte Bruksanvisning Spruta Utilização Seringa Brugsanvisning Sprøjte Χειρουργική συγκολλητική BioGlue® Cerrahi Chirurgické adhezivum BioGlue® ουσία BioGlue® Οδηγίες Yapışkan Kullanım BioGlue® Návod k použití Chirurgische Kleefstof χρήσης Σύριγγα Talimatları Şırınga Stříkačka Gebruiksinstructies Spuit Chirurgické Lepidlo BioGlue® Sebészi Klej Chirurgiczny Хирургический клей BioGlue® Návod na Szövetragasztó BioGlue® Sposób Użycia BioGlue® Инструкция по Použitie Striekačka Használati útmutató Strzykawka применению Шприц Fecskendő CryoLife, Inc. 1655 Roberts Boulevard, NW • Kennesaw, Georgia 30144 • USA • +1 (770) 419-3355 • Fax +1 (770) 590-3753 http://www.cryolife.com 25°C 0°C L6313.009 Rev (07/2010) ENGLISH PRODUCT DESCRIPTION BioGlue® Surgical Adhesive is composed of bovine serum albumin and glutaraldehyde. The product’s two components are mixed within the applicator tip of the syringe during application to tissue. Polymerization of the surgical adhesive begins immediately upon application, reaching bonding strength within 2 minutes. INDICATIONS • Do not peel away BioGlue from an unintended site, as 3. While firmly grasping the syringe, nose upward, BioGlue Surgical Adhesive is indicated for use as an this could result in tissue damage. turn the cap 90° counterclockwise and remove the adjunct to standard methods of surgical repair (such • An animal study12 has shown that the circumferential cap by rocking it from side-to-side. Align the tip with as sutures, staples, electrocautery, and/or patches) to application of BioGlue restricts dilatation on growing the syringe using the corresponding notches on bond, seal, and/or reinforce soft tissue.