Integrated Post Mda Coverage Survey Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
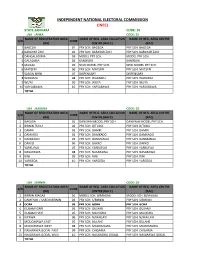
Zamfara Code: 36 Lga : Anka Code: 01 Name of Registration Area Name of Reg
INDEPENDENT NATIONAL ELECTORAL COMMISSION (INEC) STATE ZAMFARA CODE: 36 LGA : ANKA CODE: 01 NAME OF REGISTRATION AREA NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE (RA) CENTRE (RACC) (RAC) 1 BAGEGA 01 PRY SCH. BAGEGA PRY SCH. BAGEGA 2 BARAYAR ZAKI 02 PRY SCH. BARAYAR ZAKI PRY SCH. BARAYAR ZAKI 3 DANGALADIMA 03 MODEL PRY SCH. MODEL PRY SCH. 4 GALADIMA 04 KANWURI KANWURI 5 MAGAJI 05 NEW MODEL PRY SCH. NEW MODEL PRY SCH. 6 MATSERI 06 PRY SCH. MATSERI PRY SCH. MATSERI 7 SABON BIRNI 07 DISPENSARY DISPENSARY 8 WARAMU 08 PRY SCH. WARAMU PRY SCH. WARAMU 9 WUYA 09 PRY SCH. WUYA PRY SCH. WUYA 10 YAR SABAWA 10 PRY SCH. YARSABAWA PRY SCH. YARSABAWA TOTAL LGA : BAKURA CODE: 02 NAME OF REGISTRATION AREA NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE (RA) CENTRE (RACC) (RAC) 1 BAKURA 01 DANKWAI MODEL PRY SCH. DANKWAI MODEL PRY SCH. 2 BIRNIN TUDU 02 PRY SCH. B/TUDU PRY SCH. B/TUDU 3 DAMRI 03 PRY SCH. DAMRI PRY SCH. DAMRI 4 DANKADU 04 PRY SCH. DAMKADO PRY SCH. DAMKADO 5 DANMANO 05 PRY SCH. DANMANAU PRY SCH. DANMANAU 6 DAKKO 06 PRY SCH. DAKKO PRY SCH. DAKKO 7 YARKUFOJI 07 PRY SCH. YARKUFOJI PRY SCH. YARKUFOJI 8 NASARAWA 08 PRY SCH. NASARAWA PRY SCH. NASARAWA 9 RINI 09 PRY SCH. RINI PRY SCH. RINI 10 YARGEDA 10 PRY SCH. YARGEDA PRY SCH. YARGEDA TOTAL LGA : BIRNIN CODE: 03 NAME OF REGISTRATION AREA NAME OF REG. AREA COLLATION NAME OF REG. -

IOM Nigeria DTM Flash Report NCNW 26 June 2020
FLASH REPORT: POPULATION DISPLACEMENT DTM North West/North Central Nigeria. Nigeria 22 - 26 JUNE 2020 Aected Population: Casualties: Movement Trigger: 2,349 Individuals 3 Individuals Armed attacks OVERVIEW Maikwama 219 The crisis in Nigeria’s North Central and North West zones, which involves long-standing Dandume tensions between ethnic and linguis�c groups; a�acks by criminal groups; and banditry/hirabah (such as kidnapping and grand larceny along major highways) led to fresh wave of popula�on displacement. Kaita Mashi Mai'adua Jibia Shinkafi Katsina Daura Zango Dutsi Batagarawa Mani Safana Latest a�acks affected 2,349 individuals, includ- Zurmi Rimi Batsari Baure Maradun Bindawa Kurfi ing 18 injuries and 3 fatali�es, in Dandume LGA Bakura Charanchi Ingawa Jigawa Kaura Namoda Sandamu Katsina Birnin Magaji Kusada Dutsin-Ma Kankia (Katsina) and Bukkuyum LGA (Zamfara) between Talata Mafara Bungudu Matazu Dan Musa 22 - 26 June, 2020. The a�acks caused people to Gusau Zamfara Musawa Gummi Kankara flee to neighboring locali�es. Bukkuyum Anka Tsafe Malumfashi Kano Faskari Kafur Gusau Bakori A rapid assessment was conducted by field staff Maru Funtua Dandume Danja to assess the impact on people and immediate Sabuwa needs. ± GENDER (FIG. 1) Kaduna X Affected PopulationPlateau 42% Kyaram 58% Male State Bukkuyum 2,130 Female Federal Capital Territory LGA Nasarawa Affected LGAs The map is for illustration purposes only. The depiction and use of boundaries, geographic names and related data shown are not warranted to be error free nor do they imply judgment on the legal status of any territory, or any endorsement or accpetance of such boundaries by MOST NEEDED ASSISTANCE (FIG. -

Delineation of Potential Mineral Zones from Aeromagnetic Data Over Eastern Part of Zamfara
Delineation of Potential Mineral… Savanna Journal of Basic and Applied Sciences Available online at http://www.sjbas.com.ng Savanna Journal of Basic and Applied Sciences (June, 2021), 3(1): 60 - 66 P:ISSN 2695-2335 | E:ISSN 2705-3164 Delineation of Potential Mineral Zones from Aeromagnetic Data over Eastern Part of Zamfara Augie, A.I. and Ridwan, M.M. Applied Geophysics Department, Federal University Birnin Kebbi, Nigeria ABSTRACT In this study, acquired aeromagnetic data was used to delineate structures that may host minerals over the eastern part of Zamfara State. The acquired aeromagnetic data were analysed, processed and interpreted using the following techniques; IGRF/DGRF computation, reduction to magnetic equator (RTE), geologic contact (GC), centre for exploration targeting (CET) and source parameter imaging (SPI). The result from these techniques revealed the presence of granite, migmatite, biotite, gneiss, diorite, medium coarse grained quartz-mica schist and biotite- homblende granite as compared with geology of the area. Magnetic anomalous zones identified in these area were associated with the Fe-bearing mineral and sediments with widest variation in Fe- content that form various silicate and oxide mineral species which may probably formed during the formation of both intrusive and extrusive igneous rocks. These regions also have revealed the lineaments that were trending in South-East of the study area within the Birnin Magaji/Kiyawa, eastern part of Bungudu, southern part of Gusau, NE part of Tsafe and western part of Faskari. These structures were associated with hydrothermal solutions of the host rocks that may form mineral and the types of rocks/or structures within the zones play an important role in solid minerals exploration. -

Seroprevalence of Lymphatic Filariasis in Six Communities of Bungudu Lga, Zamfara State, Nigeria
Available online at www.ijpab.com Ladan et al Int. J. Pure App. Biosci. 6 (3): 11-18 (2018) ISSN: 2320 – 7051 DOI: http://dx.doi.org/10.18782/2320-7051.6568 ISSN: 2320 – 7051 Int. J. Pure App. Biosci. 6 (3): 11-18 (2018) Research Article Seroprevalence of Lymphatic Filariasis in Six Communities of Bungudu Lga, Zamfara State, Nigeria Mu’awiyya Umar Ladan2*, Tukur Adamu1 and Sirajo Isma’ila Moyi2 1Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto State, Nigeria 2Department of Biological Sciences, Federal University Gusau, Zamfara State, Nigeria *Corresponding Author E-mail: [email protected] Received: 25.05.2018 | Revised: 22.06.2018 | Accepted: 28.06.2018 ABSTRACT This study was conducted to determine the seroprevalence of lymphatic filariasis in six communities of Bungudu Local Government Area, Zamfara State, Nigeria. Blood samples from 501 volunteers were diagnosed for bancroftian antibodies using rapid diagnostic test (RDT) kits (filariasis IgG/IgM Combo). An overall prevalence of 38.72% was recorded. Rawayya-Bela ward was found to have the highest prevalence (45.24%) of infection among the wards, while least prevalence (27.38%) of infection was recorded in Kwatarkwashi ward. With respect to gender, the highest prevalence (48.46%) of infection occurred in males as against their female counterparts. The age-related prevalence showed people at age bracket 10-19 to be the highest infected (50.57%) group while those at age bracket 0-9 had the least prevalence (33.75%) of infection. With respect to occupation of the inhabitants, farmers were found to have the highest prevalence (45.61%) of infection than the other occupational groups. -

Zamfara State Universal Basic Education Board, Gusau
ZAMFARA STATE UNIVERSAL BASIC EDUCATION BOARD, GUSAU. PART A: REPORTS ON THE CONDITION OF ICT CENTERS. 1.0 INTRODUCTION: In an effort to promote computer literacy in school, Zamfara state universal basic education board established 41 school based ICT centers. The centers are equipped with 20 sets of computers, which includes the Monitors, CPU, Keyboards, Mouse, Printer and a generating set. In addition, the federal Government, under the UBEC established 7 Almajiri schools and equipped with 2 each with sets of computers. 2.0 OBJECTIVES: To assess the condition of the center facilities To track the functionality of the center operators To assess the literacy benefits of the centers To achieve the objective, the executive chairman set 21 members committee to track the condition, functionality and effectiveness of the centers and its operators. The committee has the following Term of Reference. Determine the names and ID numbers of the present ICT instructors in each center Determine if the facilities in the center is being use by the teachers and the pupils Determine who finance the fueling of generator at the ICT center Assess the conduct of the teaching and learning process at the Almajiri schools. Assess the attendance of non-Arabic/ Islamic teachers at the Almajiri schools Assess the condition of facilities at the centers. 1 The teams are distributed as below: S/ LGEAs CENTERs MEMBERS N 1 Gusau a. Iman Da’ara Almajiri Mohd & school,Tsunami area, Gusau Matusgi Tsafe b. Lman Balarabe Zawayya Binta Almajiri school, Abarma Ayuba c. Malam Yahuza Almajiri school, Lawali Gusau Gurbi d. Almajiri school, Tsafe e. -

(SQUEAC) Birnin Magaji LGA's CMAM Programme Zamfara State, Northern Nigeri
Semi-Quantitative Evaluation of Access and Coverage (SQUEAC) Birnin Magaji LGA’s CMAM programme Zamfara State, Northern Nigeria June 2014 Adamu Abubakar Yerima, Ayobami Oyedeji, Salisu Sharif Jikamshi and Ode Okponya Ode Save the Children International Acknowledgement The SQUEAC assessment in Birnin Magaji LGA was accomplished through the generous support of Children Investment Fund Foundation (CIFF). Special thank goes to the Federal Ministry of Health (FMOH), Zamfara State Ministry of Health (SMOH), Birnin Magaji Local Government Area (LGA) and Birnin Magaji LGA Health Facilities’ Staff and of prominent note, the Permanent Secretary, Director Primary Health Care & State Nutrition Officer of Zamfara State (SMOH), the Chairman and Director PHC of Birnin Magaji LGA for their collaboration in the implementation of the SQUEAC1 assessment in the state. Our profound gratitude goes to the care givers and various community leaders for sparing their precious time and opening their doors to the SQUEAC team without which this investigation would not have been a reality. Finally and most importantly, we wish to appreciate the technical support of Adaeze Oramalu (Nutrition Advisor) and Lindsey Pexton (Senior Nutrition Adviser, Save the Children International), Joseph Njau of ACF International and Coverage Monitoring Network (CMN) in analyzing the data and compilation of this report. 1 Semi Quantitative Evaluation of Access and Coverage ii Table of contents Abbreviations .............................................................................................................................................................. -

States and Lcdas Codes.Cdr
PFA CODES 28 UKANEFUN KPK AK 6 CHIBOK CBK BO 8 ETSAKO-EAST AGD ED 20 ONUIMO KWE IM 32 RIMIN-GADO RMG KN KWARA 9 IJEBU-NORTH JGB OG 30 OYO-EAST YYY OY YOBE 1 Stanbic IBTC Pension Managers Limited 0021 29 URU OFFONG ORUKO UFG AK 7 DAMBOA DAM BO 9 ETSAKO-WEST AUC ED 21 ORLU RLU IM 33 ROGO RGG KN S/N LGA NAME LGA STATE 10 IJEBU-NORTH-EAST JNE OG 31 SAKI-EAST GMD OY S/N LGA NAME LGA STATE 2 Premium Pension Limited 0022 30 URUAN DUU AK 8 DIKWA DKW BO 10 IGUEBEN GUE ED 22 ORSU AWT IM 34 SHANONO SNN KN CODE CODE 11 IJEBU-ODE JBD OG 32 SAKI-WEST SHK OY CODE CODE 3 Leadway Pensure PFA Limited 0023 31 UYO UYY AK 9 GUBIO GUB BO 11 IKPOBA-OKHA DGE ED 23 ORU-EAST MMA IM 35 SUMAILA SML KN 1 ASA AFN KW 12 IKENNE KNN OG 33 SURULERE RSD OY 1 BADE GSH YB 4 Sigma Pensions Limited 0024 10 GUZAMALA GZM BO 12 OREDO BEN ED 24 ORU-WEST NGB IM 36 TAKAI TAK KN 2 BARUTEN KSB KW 13 IMEKO-AFON MEK OG 2 BOSARI DPH YB 5 Pensions Alliance Limited 0025 ANAMBRA 11 GWOZA GZA BO 13 ORHIONMWON ABD ED 25 OWERRI-MUNICIPAL WER IM 37 TARAUNI TRN KN 3 EDU LAF KW 14 IPOKIA PKA OG PLATEAU 3 DAMATURU DTR YB 6 ARM Pension Managers Limited 0026 S/N LGA NAME LGA STATE 12 HAWUL HWL BO 14 OVIA-NORTH-EAST AKA ED 26 26 OWERRI-NORTH RRT IM 38 TOFA TEA KN 4 EKITI ARP KW 15 OBAFEMI OWODE WDE OG S/N LGA NAME LGA STATE 4 FIKA FKA YB 7 Trustfund Pensions Plc 0028 CODE CODE 13 JERE JRE BO 15 OVIA-SOUTH-WEST GBZ ED 27 27 OWERRI-WEST UMG IM 39 TSANYAWA TYW KN 5 IFELODUN SHA KW 16 ODEDAH DED OG CODE CODE 5 FUNE FUN YB 8 First Guarantee Pension Limited 0029 1 AGUATA AGU AN 14 KAGA KGG BO 16 OWAN-EAST -

Independent National Electoral Commission (INEC)
FEDERAL REPUBLIC OF NIGERIA Independent National Electoral Commission (INEC) ZAMFARA STATE DIRECTORY OF POLLING UNITS Revised January 2015 DISCLAIMER The contents of this Directory should not be referred to as a legal or administrative document for the purpose of administrative boundary or political claims. Any error of omission or inclusion found should be brought to the attention of the Independent National Electoral Commission. INEC Nigeria Directory of Polling Units Revised January 2015 Page i Table of Contents Pages Disclaimer............................................................................... i Table of Contents ……………………………………………… ii Foreword................................................................................. iv Acknowledgement................................................................... v Summary of Polling Units........................................................ 1 LOCAL GOVERNMENT AREAS Anka............................................................................... 2-7 Bakura........................................................................... 8-13 Birnin-Magaji/Kiyaw....................................................... 14-19 Bukkuyum...................................................................... 20-27 Bungudu........................................................................ 28-38 Gummi........................................................................... 39-46 Gusau............................................................................ 47-60 Kaura Namoda............................................................. -

Seasonal Malaria Chemoprevention in Nigeria: Coverage Surveys 2017
Seasonal Malaria Chemoprevention in Nigeria: Coverage surveys 2017 Report compiled by Paul Milligan, London School of Hygiene & Tropical Medicine Citation: P Milligan, M Kana, S Lal, P Snell (2018) Seasonal Malaria Chemoprevention in Nigeria: Coverage surveys 2017. Acknowledgments: D Moroso, A Roca, Malaria Consortium Page 1 of 50 Contents Summary of findings ............................................................................................................................... 5 Background ......................................................................................................................................... 5 Percentage of children who received SMC ......................................................................................... 5 Results for cycle 1 ............................................................................................................................... 6 Results for cycles 2, 3 and 4 ................................................................................................................ 6 Awareness of the dates of SMC campaigns ........................................................................................ 6 Equitability of SMC distribution .......................................................................................................... 6 Caregiver’s understanding of SMC ...................................................................................................... 7 Adherence of CHWs to guidelines for SMC administration, as reported by caregivers -

1Bfebruary to September 2019
NIGERIA Food Security Outlook February to September 2019 Intense insurgent attacks in northeast Nigeria lead to increased displacement and food needs KEY MESSAGES • Conflict in northeast Nigeria continues to threaten lives Current food security outcomes, February 2019 and livelihoods. Affected resident and displaced populations continue to face significant difficulty meeting their basic food needs, and worst-affected areas are likely in Emergency (IPC Phase 4). Inaccessible areas are likely facing similar or worse food security outcomes as neighboring, accessible areas. More extreme levels of acute food insecurity are possible in a worst-case scenario where displaced populations become cutoff due to a shift in conflict and emergency assistance provision is halted. • Many households continue to be impacted by farmer/herder conflict in central and northern states, and affected populations remain displaced in Zamfara, Source: FEWS NET Katsina, Kaduna, Taraba, Plateau, Benue, Nasarawa, and Adamawa states. The conflict keeps households from engaging in normal livelihood activities in affected areas. Larger populations have restricted access to market and income opportunities in Zamfara and Katsina states and will likely be Stressed (IPC Phase 2) through September 2018. • In much of the rest of the country, households are consuming own food stocks in the postharvest period. Households are engaged normally in dry season activities, petty trading, and labor work to earn income. Staple food prices are generally exhibiting typical trends and will peak during the lean season period between July and September. Consequently, most households will face Minimal (IPC Phase 1) acute food insecurity between February and September 2019 across the country. NATIONAL OVERVIEW Current Situation Dry season activities: Dry season cultivation started normally in December 2018 when waters receded in local ponds and rivers. -

Seasonal Malaria Chemoprevention Survey in Northern Nigeria 2018
SEASONAL MALARIA CHEMOPREVENTION SURVEY IN NORTHERN NIGERIA 2018 Final Report OPM Nigeria January 2019 Seasonal Malaria Chemoprevention Survey in Northern Nigeria 2018 Table of Contents Table of Contents 2 List of tables and figures 3 Acknowledgement 4 List of Abbreviation 5 Executive Summary 6 1 Introduction 8 1.1 Overview of Malaria Consortium’s Seasonal Malaria Chemoprevention (SMC) Programme 8 1.2 Rational of the SMC Programme 9 2 Methodology 10 2.1 Sampling Methodology 10 2.2 Sample Size Determination 12 2.3 Sampling Strategy 12 2.4 Area covered by the survey 13 2.5 Data Collection and Management 14 2.6 Ethical Approval and Confidentiality of Data Sets 15 2.7 Survey instruments 15 3 Results 17 3.1 Survey completion rate 17 3.2 SMC Coverage by Cycle 20 3.3 Understanding of SMC card use 24 3.4 Non-Treatment 25 3.5 General Health Concerns and Behaviours 26 3.6 General Adverse Reaction 29 3.7 SMC Card Usage and Retention 31 3.8 Treatment with the DOT by CHW and Caregivers 32 3.9 Caregiver Knowledge and Perception 33 3.10 General Knowledge and Behaviour related to SMC 34 4 Conclusion and Recommendation 35 Annex A Other Tables 38 Annex B List of Facilities and Communities Replaced 39 Annex C OPM Survey Management Team 51 Annex D Questionnaire 52 © Oxford Policy Management 2 Seasonal Malaria Chemoprevention Survey in Northern Nigeria 2018 List of tables and figures Table 1: Distribution of interviews across study sites .................................................................................. 17 Table 2: Age distribution of children in the survey ...................................................................................... -

Nigeria, First Quarter 2020: Update on Incidents According to the Armed Conflict Location & Event Data Project
NIGERIA, FIRST QUARTER 2020: Update on incidents according to the Armed Conflict Location & Event Data Project (ACLED) compiled by ACCORD, 23 June 2020 Number of reported incidents with at least one fatality Number of reported fatalities National borders: GADM, November 2015a; administrative divisions: GADM, November 2015b; in- cident data: ACLED, 20 June 2020; coastlines and inland waters: Smith and Wessel, 1 May 2015 NIGERIA, FIRST QUARTER 2020: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) COMPILED BY ACCORD, 23 JUNE 2020 Contents Conflict incidents by category Number of Number of reported fatalities 1 Number of Number of Category incidents with at incidents fatalities Number of reported incidents with at least one fatality 1 least one fatality Violence against civilians 213 139 562 Conflict incidents by category 2 Protests 199 0 0 Development of conflict incidents from March 2018 to March 2020 2 Battles 151 113 510 Riots 62 25 52 Methodology 3 Explosions / Remote 38 30 285 Conflict incidents per province 4 violence Strategic developments 27 0 0 Localization of conflict incidents 4 Total 690 307 1409 Disclaimer 7 This table is based on data from ACLED (datasets used: ACLED, 20 June 2020). Development of conflict incidents from March 2018 to March 2020 This graph is based on data from ACLED (datasets used: ACLED, 20 June 2020). 2 NIGERIA, FIRST QUARTER 2020: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) COMPILED BY ACCORD, 23 JUNE 2020 Methodology on what level of detail is reported. Thus, towns may represent the wider region in which an incident occured, or the provincial capital may be used if only the province The data used in this report was collected by the Armed Conflict Location & Event is known.