Predation at a Snail's Pace. What Is Needed for a Successful Hunt?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EAZA Best Practice Guidelines for Polynesian Tree Snails (Partula Spp)
EAZA Best Practice Guidelines for Polynesian tree snails (Partula spp) Edition 1.0 Publication date June 2019 Partula Snail EEP Species Committee Editor Dave Clarke, ZSL 2019_Partula sp_EAZA Best Practice Guidelines EAZA Best Practice Guidelines for Polynesian tree snails (Partula spp) Terrestrial Invertebrate Taxon Advisory Group TITAG Chair: Mark Bushell, Bristol Zoo Gardens, Clifton, Bristol, BS8 3HA [email protected] TITAG Vice-Chairs: Tamás Papp, Chester Zoo, Moston Rd, Upton, Chester CH2 1EU. [email protected] & Vítek Lukáš, Zoo Praha, U Trojského zámku 3/120, 171 00 Praha 7, Czechia. [email protected] EEP Co-ordinator: Paul Pearce-Kelly, ZSL [email protected] EEP Studbook keeper: Sam Aberdeen, ZSL [email protected] Edition 1.0 Publication date June 2019 (based on global Management Guidelines document Nov 2007 eds Pearce-Kelly, Blake, Goellner & Snider) Editor Dave Clarke, ZSL [email protected] Citation - Clarke, D., EAZA Best Practice Guidelines for Partula snails. EAZA 2019 We acknowledge the invaluable input of all Partula snail EEP Species Committee members, SSP colleagues and global participating Partula collections. EAZA Best Practice Guidelines disclaimer Copyright (June 2019) by EAZA Executive Office, Amsterdam. All rights reserved. No part of this publication may be reproduced in hard copy, machine-readable or other forms without advance written permission from the European Association of Zoos and Aquaria (EAZA). Members of the European Association of Zoos and Aquaria (EAZA) may copy this information for their own use as needed. The information contained in these EAZA Best Practice Guidelines has been obtained from numerous sources believed to be reliable. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Copy 3 of Janszoon Toolboxes
Invertebrates in the Abel Tasman National Park The terrestrial biodiversity in the Abel Tasman National Park is awesome! Listed below are some of the species commonly found in the park. See the species identification pages to find out more about each one. Also look for the instructions on how to attract invertebrates into your garden with wētā motels and wooden disks. Invertebrates About 97% of all known animals are invertebrates! Invertebrates are animals without a backbone/spine and are the most varied group of animals on the planet. Most invertebrates in New Zealand are found nowhere else in the world and there are thousands of invertebrates species in New Zealand. Invertebrates are an important part of ecosystems to help to keep the balance in nature in numerous ways. They are responsible for pollinating plants, recycling nutrientsSOCIAL and MEDIA keeping population of other living things stable. Some break FOLLOWERSdown pollutants, build and maintain soils and deal with natural waste. Invertebrates are an important food source for many animals such asVISITORS native birds, frogs, lizards, fish and bats. Some invertebrates also eat other invertebrates (e.g. dragonflies eat mosquitoes). Without invertebratesVENDORS ecosystems AND could not survive: they are essential for a healthy environmentEXHIBITORS . In the Abel Tasman National ParkMEDIA we see ATENDEES invertebrates such as: wētā, snails - powelliphanta hochstetteri & rhytida oconnori, giant earthworm, leaf-veined slug & cicadas. Image: Experiencing invertebrates in your green spaces, p26, Department of Conservation, te papa atawhai Rhytida oconnori Habitat New Zealand rhytida snails typically live under fern or leaf litter and in damp rock piles in unmodified native forest, and under tussock or scrub in the subalpine zone. -

Husbandry of the Carnivorous Land Snail, Powelliphanta Augusta (Gastropoda: Pulmonata: Rhytdidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ResearchArchive at Victoria University of Wellington Husbandry of the Carnivorous Land Snail, Powelliphanta augusta (Gastropoda: Pulmonata: Rhytdidae) By Thomas Edward Allan A thesis submitted to the Victoria University of Wellington in fulfillment of the requirements for the degree of Master of Science in Ecological Restoration Victoria University of Wellington 2010 1 Abstract Key aspects of the captive husbandry of Powelliphanta augusta, a newly-described New Zealand land snail are investigated: how they should be managed and fed to provide individuals for release, and how a long-term captive population can be maintained as an insurance against extinction in the wild. This project arises from almost all members of this species having been brought into captivity due to their displacement in the wild by an opencast coalmine. Powelliphanta (F: Rhytididae) is a genus of endemic carnivorous snails, which includes 10 species, 27 subspecies and numerous undescribed taxa. As well as its diversity, Powelliphanta is renowned for the large size of its members (up to 90mm diameter) and their attractively-patterned shells. Most taxa are threatened due to habitat loss and predation by introduced mammalian predators. The study commences with a literature review to refine husbandry methods and to assess requirements for captive breeding of snails. From this review investigations are made into stocking densities, substrate, reproductive biology, body condition and growth of the P. augusta captive population. To determine an appropriate stocking density for P. augusta groups of six snails were kept at two densities; with either 720cm2, or 1440cm2 per group. -

Evaluating the Effects of Biogeography and Fragmentation on The
Evaluating the effects of biogeography and fragmentation on the taxonomic, functional, and genetic diversity of forest-utilising bats in a South African biodiversity hotspot by Monika Ilka Moir Dissertation presented for the degree of Doctor of Philosophy in the Faculty of Science at Stellenbosch University Supervisors: Dr. Ramugondo V. Rambau Prof. Michael I. Cherry Dr. Leigh R. Richards December 2020 i Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights, and that I have not previously in its entirety or in part submitted it for obtaining any qualification. This dissertation includes one original paper published in a peer-reviewed journal with me as lead author, and three articles submitted and under peer-review. The development and writing of the papers (published and unpublished) were the principal responsibility of myself. Monika Ilka Moir August 2020 Copyright © 2020 Stellenbosch University All rights reserved ii Stellenbosch University https://scholar.sun.ac.za Abstract Bats are a highly diverse mammalian order and are some of the most economically important non-domesticated vertebrates, providing many ecosystem services that contribute to the global economy. Yet, they remain a largely understudied taxon, particularly in the Eastern Cape province of South Africa, in which basic surveys of bat assemblages utilising indigenous forests are lacking. Indigenous forests constitute South Africa’s smallest and most fragmented biome yet support disproportionally high biodiversity. -

Fauna of New Zealand Website Copy 2010, Fnz.Landcareresearch.Co.Nz
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

A Phylogeny of the Cannibal Snails of Southern Africa, Genus Natalina Sensu Lato (Pulmonata: Rhytididae): Assessing Concordance Between Morphology and Molecular Data
Molecular Phylogenetics and Evolution 52 (2009) 167–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A phylogeny of the cannibal snails of southern Africa, genus Natalina sensu lato (Pulmonata: Rhytididae): Assessing concordance between morphology and molecular data Adnan Moussalli a,b,c,*, David G. Herbert a,b, Devi Stuart-Fox d a School of Biological and Conservation Sciences, University of KwaZulu-Natal, Pietermaritzburg 3206, South Africa b Department of Mollusca, Natal Museum, P. Bag 9070, Pietermaritzburg 3200, South Africa c Sciences Department, Museum Victoria, Carlton, Vic. 3053, Australia d Department of Zoology, University of Melbourne, Melbourne, Vic. 3010, Australia article info abstract Article history: The genus Natalina Pilsbry, 1893 is a southern African endemic belonging to the Gondwanan family of Received 12 October 2008 carnivorous snails, Rhytididae. We present a well-resolved molecular phylogeny of the genus based on Revised 14 January 2009 the mitochondrial 16S and COI genes and the nuclear ITS2 gene, and assess this in light of Watson’s [Wat- Accepted 20 February 2009 son, H., 1934. Natalina and other South African snails. Proc. Malacol. Soc. Lond. 21, 150–193] supra-spe- Available online 1 March 2009 cific classification via a re-examination of 23 morphological characters including features of the shell, radula, external anatomy and distal reproductive tract. Ancestral reconstruction and character mapping Keywords: based on the MK model reveals broad concordance between morphology and the molecular phylogeny Natalina 1 at the supra-specific level. Given this concordance and exceptionally deep divergences in the molecular Rhytididae Mitochondrial data, we recommend the elevation of the subgenera Natalina s.s., Afrorhytida, and Capitina to generic sta- Nuclear tus. -

Hon Eugenie Sage
Hon Eugenie Sage Minister of Conservation Minita mō Te Papa Atawhai EMBARGOED UNTIL 08:00 13 March 2019 PĀNUI PĀPĀHO MEDIA STATEMENT Mokihinui River catchment land to be added to Kahurangi National Park Conservation Minister Eugenie Sage today announced the largest addition of land to an existing national park in New Zealand’s history. A total of 64,400 hectares of conservation land in the Mokihinui River catchment on the West Coast north of Westport, including 15 km of riverbed, is being added to Kahurangi National Park. “Adding this area, roughly half the size of Auckland City, to Kahurangi is the largest addition of land to an existing national park in New Zealand’s history,” Eugenie Sage said. “National park status will ensure stronger protection of the Mokihinui area’s significant cultural, ecological, historic and recreational values. “A hydro-electric dam was proposed for the Mokihinui River in 2007. The hydro scheme attracted considerable public interest and strong opposition because of its environmental impacts. It would have flooded the Mokihinui Gorge and inundated beech-podocarp forests and significant habitats of threatened plants and wildlife such as whio/blue duck, kaka, bats and giant land snails. “A big thanks to the many New Zealanders and the Department of Conservation who spoke up for the river, its gorge, dramatic landscapes, beech-podocarp forests and set out the reasons they deserved protection from a hydro dam. “Today’s announcement is only possible because of that work and advocacy. It is why our Government can now give the Mokihinui Gorge, and the surrounding lands, forests, and mountains the strong protection that comes with being part of a national park. -
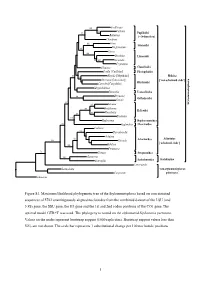
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Distribution and Status of Native Carnivorous Land Snails in The
5. Results 5.1 SUMMARY OF SPECIES DISTRIBUTIONS The distribution database contains 1187 locality records, 927 for Rhytida and 260 for Wainuia, including some duplicates. Six percent of all records are of subfossils, mostly from cave deposits. Fig. 2 shows the combined occurrence of all species by 10 000 m x 10 000 m grid square. At least one species was recorded from 406 grid squares (391 excluding subfossil records). Rhytida species were recorded in 333 squares (318 excluding subfossils) and Wainuia species in 94 squares (90 excluding subfossils). The number of 10 000 m x 10 000 m grid squares containing records of each species or subspecies ranged from 2 to 105 (Table 1). Subfossil records extended the known range to additional grid squares in 12 of the 17 taxa. Generalised range maps for all taxa are presented for quick reference in Fig. 3. The distributions are mapped in detail in Fig. 4 (see Appendix 4) and discussed in the species accounts (Appendix 4). 5.2 ASSESSMENT OF CONSERVATION STATUS Data relevant to conservation status are discussed in the species notes (Appendix 4). Direct assessment of population trends is not currently possible as no population study has been conducted for any species of Wainuia or Rhytida. A tentative priority ranking was therefore performed using the scoring system adopted for other invertebrates in Department of Conservation (1994b). The results provide a coarse and somewhat subjective indication of conservation priorities (Table 2). They can be criticised for failing to adequately incorporate uncertainty in our present knowledge, and for not identifying critically threatened populations within some species or subspecies. -

Distribution and Status of Native Carnivorous Land Snails in the Genera Wainuia and Rhytida
Distribution and status of native carnivorous land snails in the genera Wainuia and Rhytida SCIENCE FOR CONSERVATION: 101 Murray Efford Published by Department of Conservation P.O. Box 10-420 Wellington, New Zealand Science for Conservation presents the results of investigations by DOC staff, and by contracted science providers outside the Department of Conservation. Publications in this series are internally and externally peer reviewed. © December 1998, Department of Conservation ISSN 11732946 ISBN 0478217714 This publication originated from work done under Department of Conservation Investigation no. 790, carried out by Murray Efford, Manaaki Whenua - Landcare Research, Private Bag 1930, Dunedin. It was approved for publication by the Director, Science & Research Unit, Science Technology and Information Services, Department of Conservation, Wellington. Cataloguing in Publication Efford, M. G. Distribution and status of native carnivorous land snails in the genera Wainuia and Rhytida / Murray Efford. Wellington, N.Z. : Dept. of Conservation, 1998. 1 v. ; 30 cm. (Science for conservation, 11732946 ; 101.) Includes bibliographical references. ISBN 0478217714 1. SnailsNew Zealand. 2. Rhytididae. I. Title. II. Series: Science for conservation (Wellington, N.Z.) ; 101. 594.30993 20 zbn98112337 CONTENTS Abstract 5 1. Introduction 5 2. Background 5 2.1 Systematics 9 Species-level taxonomy 9 2.2 Previous research on Wainuia and Rhytida 12 2.3 Conservation status 12 3. Objectives 15 4. Methods 13 5. Results 14 5.1 Summary of species distributions 14 5.2 Assessment of conservation status 14 6. Discussion 14 6.1 Distribution 14 6.2 Conservation 18 General policy 18 Vulnerable populations 18 General management needs 19 7. Recommendations 20 8. -

Publication: Parkinson,P.G. 1971: the Subfossil Species of Albers (Mollusca: Paryphantidae)
Publication: Parkinson,P.G. 1971: The subfossil species of Albers (Mollusca: Paryphantidae). J. R. SOC. N.Z.: 1(1):1-6 This article has been provided by the BUGZ project and is for private use only and not for reproduction in any form etc, and we do not guarantee the quality of the scan, nor the correctness of the text layer relating to each page image. Project coordinators: Raphael Didham & Stephen Pawson Content scanning, OCR and cleanup by: Carl Wardhaugh, Katherine Wilson, Stephanie Kaefer, Chris Coleman, Muriele Rabone, Miriam Hall and James Aulsford Interface and database developed by: Mike Cochrane & Mark Fuglestad Project funded by: TFBIS (Terrestrial and Freshwater Biodiversity Information System) (The pages of the publication follow this cover sheet) Journal of the Royal Society of New Zealand, 1971, Vol. 1, No. 1, pp. 1-6, 14 figs. The Subfossil Species of Rhytida Albers (Mollusca : Paryphantidae) P. PARKINSON University of Auckland [Received by the Editor, 3 September 1970] Abstract THE known subf ossil forms of Rhytida Albers, 1850 are discussed and all are shown to be conspecific with living taxa: R. spelaea Powell, 1933 is synonymised with R. greenwoodi (Gray, 1850) ; R. yaldwyni Dell, 1955 is synonymised with Wainuia urnula urnula (Pfeiffer, 1855); R. hadfieldi Powell, 1949 is synonymised with R. oconnori Powell, 1946 and R. duplicata vivens Powell, 1946 is synonymised with R. duplicata duplicata Suter, 1904. INTRODUCTION TWENTY-ONE taxa in the genus Rhytida are currently recognised as New Zealand endemics. The taxonomic arrangement of these is unsatisfactory, but before a thorough revision of the group can be carried out, much work on the soft part anatomy must be done.