Use of Anthrax Vaccine in the United States Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amerithrax Investigative Summary
The United States Department of Justice AMERITHRAX INVESTIGATIVE SUMMARY Released Pursuant to the Freedom of Information Act Friday, February 19, 2010 TABLE OF CONTENTS I. THE ANTHRAX LETTER ATTACKS . .1 II. EXECUTIVE SUMMARY . 4 A. Overview of the Amerithrax Investigation . .4 B. The Elimination of Dr. Steven J. Hatfill as a Suspect . .6 C. Summary of the Investigation of Dr. Bruce E. Ivins . 6 D. Summary of Evidence from the Investigation Implicating Dr. Ivins . .8 III. THE AMERITHRAX INVESTIGATION . 11 A. Introduction . .11 B. The Investigation Prior to the Scientific Conclusions in 2007 . 12 1. Early investigation of the letters and envelopes . .12 2. Preliminary scientific testing of the Bacillus anthracis spore powder . .13 3. Early scientific findings and conclusions . .14 4. Continuing investigative efforts . 16 5. Assessing individual suspects . .17 6. Dr. Steven J. Hatfill . .19 7. Simultaneous investigative initiatives . .21 C. The Genetic Analysis . .23 IV. THE EVIDENCE AGAINST DR. BRUCE E. IVINS . 25 A. Introduction . .25 B. Background of Dr. Ivins . .25 C. Opportunity, Access and Ability . 26 1. The creation of RMR-1029 – Dr. Ivins’s flask . .26 2. RMR-1029 is the source of the murder weapon . 28 3. Dr. Ivins’s suspicious lab hours just before each mailing . .29 4. Others with access to RMR-1029 have been ruled out . .33 5. Dr. Ivins’s considerable skill and familiarity with the necessary equipment . 36 D. Motive . .38 1. Dr. Ivins’s life’s work appeared destined for failure, absent an unexpected event . .39 2. Dr. Ivins was being subjected to increasing public criticism for his work . -

Investigation of Anthrax Associated with Intentional Exposure
October 19, 2001 / Vol. 50 / No. 41 889 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 893 Recognition of Illness Associated with the Intentional Release of a Biologic Agent 897 Weekly Update: West Nile Virus Activity — United States, October 10–16, 2001 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 On October 4, 2001, CDC and state and local public health authorities reported a case of inhalational anthrax in Florida (1 ). Additional cases of anthrax subsequently have been reported from Florida and New York City. This report updates the findings of these case investigations, which indicate that infections were caused by the intentional release of Bacillus anthracis. This report also includes interim guidelines for postexposure pro- phylaxis for prevention of inhalational anthrax and other information to assist epidemi- ologists, clinicians, and laboratorians responding to intentional anthrax exposures. For these investigations, a confirmed case of anthrax was defined as 1) a clinically compatible case of cutaneous, inhalational, or gastrointestinal illness* that is laboratory confirmed by isolation of B. anthracis from an affected tissue or site or 2) other labora- tory evidence of B. anthracis infection based on at least two supportive laboratory tests. A suspected case was defined as 1) a clinically compatible case of illness without isola- tion of B. anthracis and no alternative diagnosis, but with laboratory evidence of B. anthracis by one supportive laboratory test or 2) a clinically compatible case of an- thrax epidemiologically linked to a confirmed environmental exposure, but without cor- roborative laboratory evidence of B. -

Hawaii State Department of Health Tetanus Factsheet
TETANUS ABOUT THIS DISEASE Tetanus is an infection caused by a bacterium called Clostridium tetani. Spores of tetanus bacteria are everywhere in the environment, including soil, dust, and manure. These spores develop into bacteria when they enter the body through breaks in the skin, usually through injuries from contaminated objects. Clostridium tetani produce a toxin (poison) that causes painful muscle contractions. Tetanus is often called “lockjaw” because the first sign is most commonly spasms of the jaw muscles. Tetanus can lead to serious health problems, including being unable to open the mouth and having trouble swallowing and breathing, possibly leading to death (10% to 20% of cases). Tetanus is uncommon in the United States, with an average of 30 reported cases each year. Nearly all cases of tetanus in the U.S. are among people who have never received a tetanus vaccine, or adults who don’t stay up to date on their 10-year booster shots. SIGNS AND SYMPTOMS Symptoms of tetanus include: • Jaw cramping • Sudden, involuntary muscle tightening (muscle spasms) – often in the stomach • Painful muscle stiffness all over the body • Trouble swallowing • Jerking or staring (seizures) • Headache • Fever and sweating • Changes in blood pressure and a fast heart rate. Serious health problems that can happen because of tetanus include: • Laryngospasm (uncontrolled/involuntary tightening of the vocal cords) • Fractures (broken bones) • Hospital-acquired infections (Infections caught by a patient during a hospital stay) • Pulmonary embolism (blockage of the main artery of the lung or one of its branches by a blood clot that has travelled from elsewhere in the body through the bloodstream) • Aspiration pneumonia (lung infection that develops by breathing in foreign materials) • Breathing difficulty, possibly leading to death (1 to 2 in 10 cases are fatal) TRANSMISSION Tetanus is different from other vaccine-preventable diseases because it does not spread from person to person. -

Safety and Immunogenicity of Capsular Polysaccharide–Tetanus Toxoid Conjugate Vaccines for Group B Streptococcal Types Ia and Ib
142 Safety and Immunogenicity of Capsular Polysaccharide±Tetanus Toxoid Conjugate Vaccines for Group B Streptococcal Types Ia and Ib Carol J. Baker, Lawrence C. Paoletti, Section of Infectious Diseases, Departments of Pediatrics and of Michael R. Wessels, Hilde-Kari Guttormsen, Microbiology and Immunology, Baylor College of Medicine, Houston, Marcia A. Rench, Melissa E. Hickman, Texas; Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, and Department of Microbiology and Molecular and Dennis L. Kasper Genetics, Harvard Medical School, Boston, Massachusetts About 40% of invasive group B streptococcal (GBS) isolates are capsular polysaccharide Downloaded from https://academic.oup.com/jid/article/179/1/142/877598 by guest on 01 October 2021 (CPS) types Ia or Ib. Because infant and maternal GBS infections may be preventable by maternal vaccination, individual GBS CPS have been coupled to tetanus toxoid (TT) to prepare vaccines with enhanced immunogenicity. Immunogenicity in rabbits and protective capacity in mice of a series of type Ia- and Ib-TT conjugates increased with the degree of polysaccharide-to-protein cross-linking. In total, 190 healthy, nonpregnant women aged 18±40 years were randomized in four trials to receive Ia- or Ib-TT conjugate (dose range, 3.75±63 mg of CPS component), uncoupled Ia or Ib CPS, or saline. All vaccines were well-tolerated. CPS-speci®c IgG serum concentrations peaked 4±8 weeks after vaccination and were signif- icantly higher in recipients of conjugated than of uncoupled CPS. Immune responses to the conjugates were dose-dependent and correlated in vitro with opsonophagocytosis. These re- sults support inclusion of Ia- and Ib-TT conjugates when formulating a multivalent GBS vaccine. -

2001 Anthrax Letters
Chapter Five: 2001 Anthrax Letters Author’s Note: The analysis and comments regarding the communication efforts described in this case study are solely those of the authors; this analysis does not represent the official position of the FDA. This case was selected because it is one of the few major federal efforts to distribute medical countermeasures in response to an acute biological incident. These events occurred more than a decade ago and represent the early stages of US biosecurity preparedness and response; however, this incident serves as an excellent illustration of the types of communication challenges expected in these scenarios. Due in part to the extended time since these events and the limited accessibility of individual communications and messages, this case study does not provide a comprehensive assessment of all communication efforts. In contrast to the previous case studies in this casebook, the FDA’s role in the 2001 anthrax response was relatively small, and as such, this analysis focuses principally on the communication efforts of the CDC and state and local public health agencies. The 2001 anthrax attacks have been studied extensively, and the myriad of internal and external assessments led to numerous changes to response and communications policies and protocols. The authors intend to use this case study as a means of highlighting communication challenges strictly within the context of this incident, not to evaluate the success or merit of changes made as a result of these events. Abstract The dissemination of Bacillus anthracis via the US Postal Service (USPS) in 2001 represented a new public health threat, the first intentional exposure to anthrax in the United States. -

ALTIMMUNE, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the fiscal year ended December 31, 2019 ☐ Transition Report under Section 13 or 15(d) of the Securities Exchange Act of 1934 For the transition period from to Commission File Number: 001-32587 ALTIMMUNE, INC. (Exact name of registrant as specified in its charter) Delaware 20-2726770 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 910 Clopper Road, Suite 201S, Gaithersburg, MD 20878 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code (240) 654-1450 Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common stock, par value $0.0001 per share ALT The NASDAQ Global Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Skin and Soft Tissue Infections Ohsuerin Bonura, MD, MCR Oregon Health & Science University Objectives
Difficult Skin and Soft tissue Infections OHSUErin Bonura, MD, MCR Oregon Health & Science University Objectives • Compare and contrast the epidemiology and clinical presentation of common skin and soft tissue diseases • State the management for skin and soft tissue infections OHSU• Differentiate true infection from infectious disease mimics of the skin Casey Casey is a 2 year old boy who presents with this rash. What is the best treatment? A. Soap and Water B. Ibuprofen, it will self OHSUresolve C. Dicloxacillin D. Mupirocin OHSUImpetigo Impetigo Epidemiology and Treatment OHSU Ellen Ellen is a 54 year old morbidly obese woman with DM, HTN and venous stasis who presented with a painful left leg and fever. She has had 3 episodes in the last 6 months. What do you recommend? A. Cefazolin followed by oral amoxicillin prophylaxis B. Vancomycin – this is likely OHSUMRSA C. Amoxicillin – this is likely erysipelas D. Clindamycin to cover staph and strep cellulitis Impetigo OHSUErysipelas Erysipelas Risk: lymphedema, stasis, obesity, paresis, DM, ETOH OHSURecurrence rate: 30% in 3 yrs Treatment: Penicillin Impetigo Erysipelas OHSUCellulitis Cellulitis • DEEPER than erysipelas • Microbiology: – 6-48hrs post op: think GAS… too early for staph (days in the making)! – Periorbital – Staph, Strep pneumoniae, GAS OHSU– Post Varicella - GAS – Skin popping – Staph + almost anything! Framework for Skin and Soft Tissue Infections (SSTIs) NONPurulent Purulent Necrotizing/Cellulitis/Erysipelas Furuncle/Carbuncle/Abscess Severe Moderate Mild Severe Moderate Mild I&D I&D I&D I&D IV Rx Oral Rx C&S C&S C&S C&S Vanc + Pip-tazo OHSUEmpiric IV Empiric MRSA Oral MRSA TMP/SMX Doxy What Are Your “Go-To” Oral Options For Non-Purulent SSTI? Amoxicillin Doxycycline OHSUCephalexin Doxycycline Trimethoprim-Sulfamethoxazole OHSU Miller LG, et al. -

TETANUS in ANIMALS — SUMMARY of KNOWLEDGE Malinovská, Z
DOI: 10.2478/fv-2020-0027 FOLIA VETERINARIA, 64, 3: 54—60, 2020 TETANUS IN ANIMALS — SUMMARY OF KNOWLEDGE Malinovská, Z., Čonková, E., Váczi, P. Department of Pharmacology and Toxicology University of Veterinary Medicine and Pharmacy in Košice, Komenského 73, 041 81 Košice Slovakia [email protected] ABSTRACT the effects of the neurotoxin. The treatment is difficult with an unclear prognosis. Tetanus is a neurologic non-transmissible disease (often fatal) of humans and other animals with a world- Key words: animal; Clostridium tetani; toxin; spasm; wide occurrence. Clostridium tetani is the spore produc- treatment ing bacillus which causes the bacterial disease. In deep penetrating wounds the spores germinate and produce a toxin called tetanospasmin. The main characteristic sign INTRODUCTION of tetanus is a spastic paralysis. A diagnosis is usually based on the clinical signs because the detection in the Of the clostridial neurotoxins, botulinum neurotoxin wound and the cultivation of C. tetani is very difficult. and tetanus neurotoxin are the most potent toxins [15]. Between animal species there is considerable variabili- Their extraordinary toxicity is caused by neurospecificity ty in the susceptibility to the bacillus. The most sensi- and metalloprotease activity, which results in the paralysis. tive animal species to the neurotoxin are horses. Sheep Tetanus is potentially a fatal disease and in some countries and cattle are less sensitive and tetanus in these animal it is still very active. Tetanus is a traumatic clostridiosis, species are less common. Tetanus in cats and dogs are an infection in humans and other animals with worldwide rare and dogs are less sensitive than cats. -

Lessons from the Anthrax Attacks Implications for US
Lessons from the Anthrax Attacks Implications for US. Bioterrorism Preparedness A Report on a National Forum on Biodefense Author David Heymart Research Assistants Srusha Ac h terb erg, L Joelle Laszld Organized by the Center for Strategic and International Studies and the Defense Threat Reduction Agency --CFr”V? --.....a DlSTRf BUTION This report ISfor official use only; distribution authorized to U S. government agencies, designated contractors, and those with an official need Contains information that may be exempt from public release under the Freedom of Information Act. exemption number 2 (5 USC 552); exemption number 3 (’lo USC 130).Approvalof the Defense Threat Reduction Agency prior to public release is requrred Contract Number OTRAM-02-C-0013 For Official Use Only About CSIS For four decades, the Center for Stravgic and Internahonal Studies (CSIS)has been dedicated 10 providing world leaders with strategic insights on-and pohcy solubons tcurrentand emergtng global lssues CSIS IS led by John J Hamre, former L S deputy secretary of defense It is guided by a board of trustees chaired by former U S senator Sam Nunn and consistlng of prominent individuals horn both the public and private sectors The CSIS staff of 190 researchers and support staff focus pnrnardy on three subjecr areas First, CSIS addresses the fuU spectrum of new challenges to national and mternabonal security Second, it maintains resident experts on all of the world's myor geographical regions Third, it IS committed to helping to develop new methods of governance for the global age, to this end, CSIS has programs on technology and pubhc policy, International trade and finance, and energy Headquartered in Washington, D-C ,CSIS IS pnvate, bipartlsan, and tax-exempt CSIS docs not take specific policy positions, accordygly, all views expressed herein should be understood to be solely those ofthe author Spousor. -
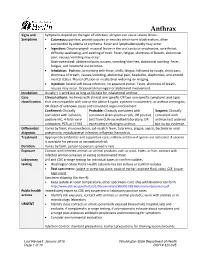
Anthrax Reporting and Investigation Guideline
Anthrax Signs and Symptoms depend on the type of infection; all types can cause severe illness: Symptoms • Cutaneous: painless, pruritic papules or vesicles which form black eschars, often surrounded by edema or erythema. Fever and lymphadenopathy may occur. • Ingestion: Oropharyngeal: mucosal lesion in the oral cavity or oropharynx, sore throat, difficulty swallowing, and swelling of neck. Fever, fatigue, shortness of breath, abdominal pain, nausea/vomiting may occur. Gastrointestinal: abdominal pain, nausea, vomiting/diarrhea, abdominal swelling. Fever, fatigue, and headache are common. • Inhalation: Biphasic, presenting with fever, chills, fatigue, followed by cough, chest pain, shortness of breath, nausea/vomiting, abdominal pain, headache, diaphoresis, and altered mental status. Pleural effusion or mediastinal widening on imaging. • Injection: Severe soft tissue infection; no apparent eschar. Fever, shortness of breath, nausea may occur. Occasional meningeal or abdominal involvement. Incubation Usually < 1 week but as long as 60 days for inhalational anthrax Case Clinical criteria: An illness with at least one specific OR two non-specific symptoms and signs classification that are compatible with one of the above 4 types, systemic involvement, or anthrax meningitis; OR death of unknown cause and consistent organ involvement Confirmed: Clinically Probable: Clinically consistent with Suspect: Clinically consistent with isolation, consistent Gram-positive rods, OR positive consistent with positive IHC, 4-fold rise in test from CLIA-accredited laboratory, OR anthrax test ordered antibodies, PCR, or LF MS epi evidence relating to anthrax but no epi evidence Differential Varies by form; mononucleosis, cat-scratch fever, tularemia, plague, sepsis, bacterial or viral diagnosis pneumonia, mycobacterial infection, influenza, hantavirus Treatment Appropriate antibiotics and supportive care; anthrax antitoxin if spores are activated. -

Version 12.0, 01 Jun 2018 ANNEX I SUMMARY of PRODUCT
BioThrax® (Anthrax Vaccine Adsorbed) 1.3.1 Summary of Product Characteristics, Labelling and Package Leaflet Version 12.0, 01 Jun 2018 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS, LABELLING AND PACKAGE LEAFLET Emergent BioSolutions, Inc. Confidential and Proprietary Page 1 of 25 BioThrax® (Anthrax Vaccine Adsorbed) 1.3.1 Summary of Product Characteristics, Labelling and Package Leaflet SUMMARY OF PRODUCT CHARACTERISTICS Emergent BioSolutions, Inc. Confidential and Proprietary Page 2 of 25 BioThrax® (Anthrax Vaccine Adsorbed) 1.3.1 Summary of Product Characteristics, Labelling and Package Leaflet This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See Section 4.8 for how to report adverse reactions 1 NAME OF THE MEDICINAL PRODUCT BioThrax1 suspension for injection. Anthrax Vaccine Adsorbed (purified cell-free filtrate) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One dose (0.5 mL) contains: Anthrax antigen filtrate: 50 micrograms (50 mcg) a, b For a full list of excipients, see Section 6.1. a Produced from cell-free filtrates of an avirulent strain of Bacillus anthracis b Adsorbed on aluminium hydroxide, hydrated (0.6 mg Al3+) 3 PHARMACEUTICAL FORM Suspension for injection. Sterile, milky-white liquid suspension, when mixed. 4 CLINICAL PARTICULARS 4.1 Therapeutic indications BioThrax is indicated for the prevention of disease caused by Bacillus anthracis, in adults at risk of exposure. BioThrax should be used in accordance with official recommendations, where available. 4.2 Posology and method of administration Posology: Primary Immunisation: 3-doses each of 0.5 mL, given at 0, 1 and 6 months. -

Immune Effector Mechanisms and Designer Vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA
EXPERT REVIEW OF VACCINES https://doi.org/10.1080/14760584.2019.1674144 REVIEW How vaccines work: immune effector mechanisms and designer vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA ABSTRACT ARTICLE HISTORY Introduction: Three major advances have led to increase in length and quality of human life: Received 6 June 2019 increased food production, improved sanitation and induction of specific adaptive immune Accepted 25 September 2019 responses to infectious agents (vaccination). Which has had the most impact is subject to debate. KEYWORDS The number and variety of infections agents and the mechanisms that they have evolved to allow Vaccines; immune effector them to colonize humans remained mysterious and confusing until the last 50 years. Since then mechanisms; toxin science has developed complex and largely successful ways to immunize against many of these neutralization; receptor infections. blockade; anaphylactic Areas covered: Six specific immune defense mechanisms have been identified. neutralization, cytolytic, reactions; antibody- immune complex, anaphylactic, T-cytotoxicity, and delayed hypersensitivity. The role of each of these mediated cytolysis; immune immune effector mechanisms in immune responses induced by vaccination against specific infectious complex reactions; T-cell- mediated cytotoxicity; agents is the subject of this review. delayed hypersensitivity Expertopinion: In the past development of specific vaccines for infections agents was largely by trial and error. With an understanding of the natural history of an infection and the effective immune response to it, one can select the method of vaccination that will elicit the appropriate immune effector mechanisms (designer vaccines). These may act to prevent infection (prevention) or eliminate an established on ongoing infection (therapeutic).