Horizontal and Vertical Distribution of Carbon Stock in Natural Stands of Hyrcanian Lowland Forests: a Case Study, Nour Forest Park, Iran
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Biological Activity and Analysis of Volatile Fraction from Pterocarya Fraxinifolia in Vegetative Stage from Iran
Journal of Sciences, Islamic Republic of Iran 28(2): 119 - 126 (2017) http://jsciences.ut.ac.ir University of Tehran, ISSN 1016-1104 Evaluation of Biological Activity and Analysis of Volatile Fraction from Pterocarya fraxinifolia in Vegetative Stage from Iran M. Akhbari*, S. Tavakoli, Z. Ghanbari, M. Dadgarnia and A. Mazoochi Essentialoils Research Institute, University of Kashan, Kashan, Islamic Republic of Iran Received: 5 June 2016 / Revised: 1 October 2016 / Accepted: 5 December 2016 Abstract This study reports chemical composition of the essential oils and in vitro antioxidant, antimicrobial and cytotoxic activity of the volatile fraction, extracted using simulations steam distillation-solvent extraction (SDE) method, and methanolic extract from the stems and young leaves of Pterocarya fraxinifolia from Gilan, west-north of Iran for the first time. The extraction yield of volatile fraction from stems was about two times more than that of leaves. GC and GC/MS analysis of the stem oil, exhibited 44 components; the most abundant constituents was hexadecanoic acid. On the other hand, 23 components were identified in the oil from leaves, with 3¸7-guaiadiene as the major components. The oils and extracts from both examined plant samples showed excellent antioxidant activities in 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay (IC50 values <45 µg/mL). In ß-carotene bleaching assay, only the extracts showed high activities with inhibition percentages more than 80%. Total phenolic contents, based on gallic acid equivalent, for the leaves and stems extracts were also very high. Screening of cytotoxic activity of the extracts via brine shrimp lethality assay exhibited higher activity for stem. -

Wingnut (Juglandaceae)
83 Wingnut (Juglandaceae) as a new generic host for Pityophthorus juglandis (Coleoptera: Curculionidae) and the thousand cankers disease pathogen, Geosmithia morbida (Ascomycota: Hypocreales) Stacy M. Hishinuma, Paul L. Dallara, Mohammad A. Yaghmour, Marcelo M. Zerillo, Corwin M. Parker, Tatiana V. Roubtsova, Tivonne L. Nguyen, Ned A. Tisserat, Richard M. Bostock, Mary L. Flint, Steven J. Seybold1 Abstract—The walnut twig beetle (WTB), Pityophthorus juglandis Blackman (Coleoptera: Curculionidae), vectors a fungus, Geosmithia morbida Kolařík, Freeland, Utley, and Tisserat (Ascomycota: Hypocreales), which colonises and kills the phloem of walnut and butternut trees, Juglans Linnaeus (Juglandaceae). Over the past two decades, this condition, known as thousand cankers disease (TCD), has led to the widespread mortality of Juglans species in the United States of America. Recently the beetle and pathogen were discovered on several Juglans species in northern Italy. Little is known about the extra-generic extent of host acceptability and suitability for the WTB. We report the occurrence of both the WTB and G. morbida in three species of wingnut, Pterocarya fraxinifolia Spach, Pterocarya rhoifolia Siebold and Zuccarini, and Pterocarya stenoptera de Candolle (Juglandaceae) growing in the United States Department of Agriculture-Agricultural Research Service, National Clonal Germplasm Repository collection in northern California (NCGR) and in the Los Angeles County Arboretum and Botanic Garden in southern California, United States of America. In two instances (once in P. stenoptera and once in P. fraxinifolia) teneral (i.e., brood) adult WTB emerged and were collected more than four months after infested branch sections had been collected in the field. Koch’s postulates were satisfied with an isolate of G. -
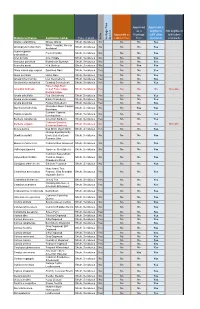
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Whole Genome Based Insights Into the Phylogeny and Evolution of the Juglandaceae
Whole Genome based Insights into the Phylogeny and Evolution of the Juglandaceae Huijuan Zhou Northwest A&F University: Northwest Agriculture and Forestry University Yiheng Hu Northwestern University Aziz Ebrahimi Purdue University Peiliang Liu Northwestern University Keith Woeste Purdue University Shuoxin Zhang Northwest A&F University: Northwest Agriculture and Forestry University Peng Zhao ( [email protected] ) Northwest University https://orcid.org/0000-0003-3033-6982 Research article Keywords: Diversication, Divergence time, Genome, Juglandaceae, Phylogenomics, Plastome Posted Date: May 24th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-495294/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/23 Abstract Background: The walnut family (Juglandaceae) contains commercially important woody trees commonly called walnut, wingnut, pecan and hickory. Phylogenetic relationships in the Juglandaceae are problematic, and their historical diversication has not been claried, in part because of low phylogenetic resolution and/or insucient marker variability. Results: We reconstructed the backbone phylogenetic relationships of Juglandaceae using organelle and nuclear genome data from 27 species. The divergence time of Juglandaceae was estimated to be 78.7 Mya. The major lineages diversied in warm and dry habitats during the mid-Paleocene and early Eocene. The plastid, mitochondrial, and nuclear phylogenetic analyses all revealed three subfamilies, i.e., Juglandoideae, Engelhardioideae, Rhoipteleoideae. Five genera of Juglandoideae were strongly supported. Juglandaceae were estimated to have originated during the late Cretaceous, while Juglandoideae were estimated to have originated during the Paleocene, with evidence for rapid diversication events during several glacial and geological periods. The phylogenetic analyses of organelle sequences and nuclear genome yielded highly supported incongruence positions for J. -

Vitis Vinifera Subsp. Sylvestris
Wild grapevine (Vitis vinifera subsp. sylvestris) in the Hyrcanian relict forests of northern Iran: an overview of current taxonomy, ecology and palaeorecords Alireza Naqinezhad, Elias Ramezani, Morteza Djamali, Annik Schnitzler, Claire Arnold To cite this version: Alireza Naqinezhad, Elias Ramezani, Morteza Djamali, Annik Schnitzler, Claire Arnold. Wild grapevine (Vitis vinifera subsp. sylvestris) in the Hyrcanian relict forests of northern Iran: an overview of current taxonomy, ecology and palaeorecords. Journal of Forestry Research, Springer Verlag, 2018, 29, pp.1757-1768. 10.1007/s11676-017-0549-6. hal-01772546 HAL Id: hal-01772546 https://hal.archives-ouvertes.fr/hal-01772546 Submitted on 9 May 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. J. For. Res. https://doi.org/10.1007/s11676-017-0549-6 ORIGINAL PAPER Wild grapevine (Vitis vinifera subsp. sylvestris) in the Hyrcanian relict forests of northern Iran: an overview of current taxonomy, ecology and palaeorecords Alireza Naqinezhad1 · Elias Ramezani2 · Morteza Djamali3 · Annik Schnitzler4 · Claire Arnold5 Received: 27 December 2016 / Accepted: 26 June 2017 © Northeast Forestry University and Springer-Verlag GmbH Germany, part of Springer Nature 2017 Abstract Due to severe anthropogenic impacts on lowland subsp. -

Diaporthales)
Persoonia 38, 2017: 136–155 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/003158517X694768 Juglanconis gen. nov. on Juglandaceae, and the new family Juglanconidaceae (Diaporthales) H. Voglmayr1, L.A. Castlebury2, W.M. Jaklitsch1,3 Key words Abstract Molecular phylogenetic analyses of ITS-LSU rDNA sequence data demonstrate that Melanconis species occurring on Juglandaceae are phylogenetically distinct from Melanconis s.str., and therefore the new genus Juglan- Ascomycota conis is described. Morphologically, the genus Juglanconis differs from Melanconis by light to dark brown conidia with Diaporthales irregular verrucae on the inner surface of the conidial wall, while in Melanconis s.str. they are smooth. Juglanconis molecular phylogeny forms a separate clade not affiliated with a described family of Diaporthales, and the family Juglanconidaceae is new species introduced to accommodate it. Data of macro- and microscopic morphology and phylogenetic multilocus analyses pathogen of partial nuSSU-ITS-LSU rDNA, cal, his, ms204, rpb1, rpb2, tef1 and tub2 sequences revealed four distinct species systematics of Juglanconis. Comparison of the markers revealed that tef1 introns are the best performing markers for species delimitation, followed by cal, ms204 and tub2. The ITS, which is the primary barcoding locus for fungi, is amongst the poorest performing markers analysed, due to the comparatively low number of informative characters. Melanconium juglandinum (= Melanconis carthusiana), M. oblongum (= Melanconis juglandis) and M. pterocaryae are formally combined into Juglanconis, and J. appendiculata is described as a new species. Melanconium juglandinum and Melanconis carthusiana are neotypified and M. oblongum and Diaporthe juglandis are lectotypified. A short descrip- tion and illustrations of the holotype of Melanconium ershadii from Pterocarya fraxinifolia are given, but based on morphology it is not considered to belong to Juglanconis. -

A New Distribution of Caucasian Wingnut (Pterocarya Fraxinifolia (Poiret) Spach) in the Kahramanmaras Region, Turkey
Journal of Environmental Biology J. Environ. Biol. ISSN: 0254-8704 25 (1),45-50 (2004) http://www.geocities.com/Lenviron_biol/ L environ [email protected] A new distribution of Caucasian wingnut (Pterocarya fraxinifolia (Poiret) Spach) in the Kahramanmaras region, Turkey Mahmut D. Avsar and Tolga Ok Department of Forest Engineering, Faculty of Forestry, Kahramanmaras Sutcu Imam University, Kahramanmaras, Turkey. (Received : 24 March, 2003 ; Accepted : 30 June, 2003) Abstract : Caucasian wingnut (Pterocarya fraxinifolia (Poiret) Spach) is a relict tree species having limited natural distribution in Turkey. In this study, a new distribution of this species in the Kahramanmaras region was explained. This distribution occurs in Onsenhopuru and Yavuzlar villages and Yesilyore town of Turkoglu district, at elevations between 600 and 640 m along Orcan stream, and continues about 4 km. In this area, Caucasian wingnut had about 100 trees. This distribution area of the species, quite important for biodiversity, should be protected and the existing individuals should be evaluated as a gene resource. Especially vegetative reproduction of the species should be started and the seedlings obtained should be used at the margins of lakes and streams, parks and large gardens, avenues, boulevards, and streets in the region. Key words : Pterocarya fraxinifolia, Natural distribution, Ecological characteristics, Biological characteristics, Protection. Introduction province were investigated, and some important The genus Pferocarya contains 8 species ecological and biological characteristics of the species throughout the world. One of these species is Caucasian were determined (Avsar, 2001). It was reported that this wingnut (Pferocarya fraxinifolia (Poiret) Spach) oecurring species also occurs in Andirin district of Kahramanmaras in Turkey, the Caucasus, and northern Iran. -

Desiccation, Dormancy, and Storage of Pterocarya Fraxinifolia
24 ARTICLE Desiccation, dormancy, and storage of Pterocarya fraxinifolia (Juglandaceae) seeds: application in Hyrcanian and Colchian forest conservation Mikołaj Krzysztof Wawrzyniak, Anna Katarzyna Jasinska,´ Paweł Chmielarz, and Gregor Kozlowski Abstract: Pterocarya fraxinifolia (Poir.) Spach (Juglandaceae) is a model relict tree species native to South Caucasus and is a typical element of threatened riparian forests. Intensive land transformations, which are common in Transcaucasia, have resulted in loss of natural habitat and population decline of the species. One of the methods of ex situ conservation is seed banking. Cryopreservation in liquid nitrogen (−196 °C) is of particular interest, as it allows safe preservation of valuable plant genetic resources. However, the feasibility of seed cryopreservation is related to the desiccation tolerance and intrinsic composition of the seeds. In this study, we examined the physiological traits of Pterocarya fraxinifolia seeds, for which desiccation tolerance is unknown or controversial, and their feasibility for cryopreservation. Additionally, we tested stratification methods for dor- mancy assessment. Results showed that seeds survived desiccation to a moisture content of 2.8% with a germination rate of 64%. Stratification at a temperature of 3 °C for 8 weeks proved to be both fast and effective. Seed moisture content ranging from 2.8% to 18.1% was determined to be safe for cryopreservation. There was no difference in seedling emergence in seeds stored for 1 year regardless of the storage temperature (−3, −18, or −196 °C). Based on our results, Pterocarya fraxinifolia seeds can be classified as orthodox. This study demonstrates for the first time the feasibility of cryopreserving Pterocarya fraxinifolia seeds. -

Gregor Aas Juglandaceae Freiburg 2016.Pdf
Morphologie und Diversität der Walnussgewächse Bayreuth (Juglandaceae) Universität Universität Garten Garten Botanischer Ökologisch- ǀ Gregor Aas Aas Gregor 20. Okt 2016 Juglans ailantifolia Juglandaceae gehören aktuell zu den Fagales Bayreuth (Buchenartige)! Nothofagaceae Universität Universität Fagaceae Myricaceae Garten Garten Juglandaceae Ordnung Fagales Rhoipteleaceae Botanischer Ticodendraceae Ökologisch- ǀ Betulaceae Casuarinaceae Gregor Aas Aas Gregor 20. Okt 2016 Kätzchenblüher! Juglandaceen: kleine, formenreiche Familie, Bayreuth 8 (9) Gattungen, 60-70 Arten. Universität Universität Garten Garten Botanischer Ökologisch- ǀ Verbreitung: v.a. nördlich temperat bis subtropisch, südlich bis Südost-Asien und Süd-Amerika Gregor Aas Aas Gregor 20. Okt 2016 Merkmale der Juglandaceen: Bayreuth - Laubabwerfende Bäume (Sträucher); - Blätter i.d.R. wechselständig, gefiedert, Universität Universität ohne Nebenblätter; - Blüten eingeschlechtig und einhäusig; - ♂ Blüten in Kätzchen an vorjährigen Trieben, - ♀ Blüten in Ähren an dies- Garten Garten jährigen Trieben; - Windbestäubung; Botanischer - Fruchtknoten aus 2 Frucht- blättern und mit 2 meist deutlichen Narben; Ökologisch- ǀ - Steinfrucht oder Nuss. Gregor Aas Aas Gregor 20. Okt Markowski 2007 2016 © System der Juglandaceae, Walnussgewächse Bayreuth Universität Universität Unterfamilie Tribus Subtribus Gattung Engelhardia 1. Engelhardioideae Oreomunnea Alfaroa Garten Garten Platycaryeae Platycarya 2. Juglandoideae Juglandeae Caryinae Carya Botanischer „Walnussartige“ Juglandinae Pterocarya -
![Pterocarya [Pdf]](https://docslib.b-cdn.net/cover/3987/pterocarya-pdf-5093987.webp)
Pterocarya [Pdf]
http://plantipodes.cat/ Pterocarya (EP, aprox. 1): 1 Generalitats i Llegenda / Generalities and Legend http://plantipodes.cat/generalitats [cccc] Resum de codis / Summary of codes (ca, aran, es, fr, en): ►▌ (al final / at the bottom) Llengües / Languages http://plantipodes.cat/lng Informació general / General information http://plantipodes.cat/generaldb Imatges externes: La seva identitat pot no ser exacta External Images: Their identity may be inaccurate Els noms científics estan formats per noms llatins o llatinitzats, se solen escriure en lletra cursiva (si el text general és en lletra rodona). La resta de components (f., var., subsp., ×, nom. illeg., nom. superfl., opus utique oppr., etc.) així com també els noms de les 'Cultivars' i dels Autors (i: &, al., auct., ex) van en la lletra normal en que s'està escrivint. Aquí, de moment, per conveniència cada registre de la base té una única mena de lletra. Scientific names are formed by Latin or Latinized names, they are usually written in italics (if the text is written in a regular type). The rest of the components (f., var., subsp., ×, name illeg., nom. superfl., opus utique oppr., etc.), as well as the names of the 'Cultivars' and the Authors (and: &, al., auct., ex) should be written in the same type as the rest of the text. Here, for the moment, for convenience, a single type of letter has been used for each record in the database. Pterocarya (aprox. 1a) 2019-07-09 nll subnll nom. Pterocarya Kunth. syn. Sp. ⊂ Fam.Pterocarya (6 sp.) ⊂ Juglandinae ⊂ Juglandeae ⊂ Juglandoideae ⊂ Juglandaceae Disb. 33 Cauc, 34 As-W, 36 Xina, 36 Tib, 38 As-E, 41 Indox, cult, cult-orn, f.v. -

Evaluation and Propagation of Chinese Wingnut, Pterocarya Stenoptera, As a New Bioenergy Feedstock
EVALUATION AND PROPAGATION OF CHINESE WINGNUT, PTEROCARYA STENOPTERA, AS A NEW BIOENERGY FEEDSTOCK BY ROBERT JOHN MILLER THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Crop Sciences in the Graduate College of the University of Illinois at Urbana-Champaign, 2014 Urbana, Illinois Advisor: Associate Professor Gary Kling ii ABSTRACT Short rotation woody crops (SRWC) as a source of biomass for energy production could play a vital role in solving the problems associated with climate change, global warming, and the rising price and diminishing supplies of carbon-based fossil fuels. In order to meet these challenges, new species will need to be assessed for use as SRWCs. Chinese wingnut, Pterocarya stenoptera, has been reported to exhibit several characteristics such as fast growth rate and suitability to a wide range of soil and moisture conditions that could make it attractive as a potential source of woody biomass. Due to lack of past work with the species, little is known regarding clonal and seed propagation, coppicing ability, biomass composition and growth rate. In order to determine the best methods of propagation for this species, hardwood and semi-hardwood cuttings were treated with a range of Indole-3-butyric acid (IBA) concentrations suspended in a mixture of 25% ethanol or suspended in talcum powder. The cuttings were subjected to wounding or non-wounding treatments and treated with liquid quick dip IBA concentrations of 1000, 2500 and 5000 ppm or mixed with talcum powder to produce concentrations of 1000, 3000, and 8000 ppm IBA. The cuttings were grown under mist conditions for 6 to 9 weeks with 14 hour photoperiods. -

Identification of Volatile Components and Biological Properties of the Extracts of Aerial Parts of Pterocarya Fraxinifolia L
Biological Forum – An International Journal 7(1): 262-266(2015) ISSN No. (Print): 0975-1130 ISSN No. (Online): 2249-3239 Identification of Volatile Components and Biological Properties of the extracts of aerial parts of Pterocarya fraxinifolia L. Zahra Aghajani*, Ali-Asghar Engashte-Vahed* and Maryam Akhbari** *Department of Chemistry, Qom Branch, Islamic Azad University, Qom, IRAN. **Essential oil research institute, University of Kashan, Kashan, IRAN. (Corresponding author: Zahra Aghajani) (Received 10 December, 2014, Accepted 01 February, 2015) (Published by Research Trend, Website: www.researchtrend.net) ABSTRACT: The volatile components of hexane extract of the leaf and stem of the Pterocarya fraxinifolia L., a native plant of the northern Iran Caspian coastal, obtained by cold percolation method and analyzed using GC/FID and GC/MS and their antioxidant and anti-bacterial activity were assessed. Palmitic acid (53.66 %) and 9, 17-Octadecadienal (22.36%) were the main components of the stem hexane extract and Aromadendrene and 9, 17-Octadecadienal in the leaves hexane extract of the plants with 27.86 % and 18.49 % were the major components respectively. The number of identified compounds in the volatile components of hexane leaves extract was 11 while the number in hexane stem extract was 9. In this research antioxidant activity of hexane and acetone extracts of leaves and stems of the P. fraxinifolia were evaluated via DPPH radical scavenging and beta-carotene bleaching assays and also the antibacterial properties of these extracts against five strains of bacteria were assessed. Keywords: Pterocarya fraxinifolia, volatile components, antibacterial activity, antioxidant activity INTRODUCTION reports by the same group and others in the same province (i.e.