Vulnerability and Hydraulic Segmentations at the Stem–Leaf Transition: Coordination Across Neotropical Trees
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphometric Leaf Variation in Oaks (Quercus) of Bolu, Turkey
Ann. Bot. Fennici 40: 233–242 ISSN 0003-3847 Helsinki 29 August 2003 © Finnish Zoological and Botanical Publishing Board 2003 Morphometric leaf variation in oaks (Quercus) of Bolu, Turkey Aydın Borazan & Mehmet T. Babaç Department of Biology, Abant |zzet Baysal University, Gölköy 14280 Bolu, Turkey (e-mail: [email protected], [email protected]) Received 16 Sep. 2002, revised version received 7 Jan. 2003, accepted 10 Jan. 2003 Borazan, A. & Babaç, M. T. 2003: Morphometric leaf variation in oaks (Quercus) of Bolu, Turkey. — Ann. Bot. Fennici 40: 233–242. Genus Quercus (Fagaceae) has a problematic taxonomy because of widespread hybridization between the infrageneric taxa. The pattern of morphological leaf varia- tion was evaluated for evidence of hybridization in Bolu, Turkey, since previous stud- ies suggested that in oaks leaf morphology is a good indicator of putative hybridiza- tion. Principal components analysis was applied to data sets of leaf characters from fi ve populations to describe variation in leaf morphology. Leaf characters analyzed in this study showed high degrees of variation as a result of hybridization between four taxa (Q. pubescens, Q. virgiliana, Q. petraea and Q. robur) of subgenus Quercus while Q. cerris as a member of subgenus Cerris was clearly separated from the others. Key words: hybridization, morphological leaf variation, principal components analy- sis, Quercus Introduction in regions of mild and warm temperate climates. Fossil leaves indicate that todayʼs several major In the northern hemisphere oaks (Quercus) are oak groups are at least 40 million years old. Gen- conspicuous members of the temperate decidu- eral distribution of fossil ancestors supports the ous, broad leaved forests. -

Platonia Insignis Instituto Interamericano De Cooperación Para La Agricultura (IICA), Edición 2018
BACURI Platonia insignis Instituto Interamericano de Cooperación para la Agricultura (IICA), Edición 2018 Este documento se encuentra bajo una Licencia Creative Commons Atribución- NoComercial-CompartirIgual 3.0 Unported. Basada en una obra en www.iica.int. El Instituto promueve el uso justo de este documento. Se solicita que sea citado apropiadamente cuando corresponda. Esta publicación está disponible en formato electrónico (PDF) en el sitio Web institucional en http://www.procisur.org.uy Coordinación editorial: Rosanna Leggiadro Corrección de estilo: Malvina Galván Diseño de portada: Esteban Grille Diseño editorial: Esteban Grille Platonia insignis Bacuri José Edmar Urano de Carvalho Walnice Maria Oliveira do Nascimento1 1. ANTECEDENTES HISTÓRICOS E CULTURAIS O bacurizeiro (Platonia insignis Mart.) é uma espécie arbórea de dupla aptidão (madeira e fruto), nativa da Amazônia Brasileira. Os frutos dessa Clusiaceae, pelo sabor e aroma agradáveis, são bastante apreciados pela população da Amazônia e de parte do Nordeste do Brasil, em particular dos Estados do Maranhão e do Piauí, onde a espécie também é encontrada espontaneamente. Em termos de sabor, o bacuri é considerado por muitos como a melhor fruta da Amazônia. Não obstante a grande demanda por essa fruta e a excelente cotação que atinge na Amazônia e nas demais áreas de ocorrência, o cultivo do bacuri- zeiro ainda é inexpressivo. Tal fato é devido às dificuldades de propagação e a longa fase jovem da planta. No início da década de 1940, Pesce (2009) já enfatizava que a planta não era cultivada, devido ao seu crescimento lento, conquanto, naquela época, os frutos eram muito procurados pelas peque- nas fábricas de doces, bastante comuns no Estado do Pará nessa época, e consumidos pela população local, ao natural e na forma de refresco, licor, doce em pasta e compota. -

What Is a Tree in the Mediterranean Basin Hotspot? a Critical Analysis
Médail et al. Forest Ecosystems (2019) 6:17 https://doi.org/10.1186/s40663-019-0170-6 RESEARCH Open Access What is a tree in the Mediterranean Basin hotspot? A critical analysis Frédéric Médail1* , Anne-Christine Monnet1, Daniel Pavon1, Toni Nikolic2, Panayotis Dimopoulos3, Gianluigi Bacchetta4, Juan Arroyo5, Zoltán Barina6, Marwan Cheikh Albassatneh7, Gianniantonio Domina8, Bruno Fady9, Vlado Matevski10, Stephen Mifsud11 and Agathe Leriche1 Abstract Background: Tree species represent 20% of the vascular plant species worldwide and they play a crucial role in the global functioning of the biosphere. The Mediterranean Basin is one of the 36 world biodiversity hotspots, and it is estimated that forests covered 82% of the landscape before the first human impacts, thousands of years ago. However, the spatial distribution of the Mediterranean biodiversity is still imperfectly known, and a focus on tree species constitutes a key issue for understanding forest functioning and develop conservation strategies. Methods: We provide the first comprehensive checklist of all native tree taxa (species and subspecies) present in the Mediterranean-European region (from Portugal to Cyprus). We identified some cases of woody species difficult to categorize as trees that we further called “cryptic trees”. We collected the occurrences of tree taxa by “administrative regions”, i.e. country or large island, and by biogeographical provinces. We studied the species-area relationship, and evaluated the conservation issues for threatened taxa following IUCN criteria. Results: We identified 245 tree taxa that included 210 species and 35 subspecies, belonging to 33 families and 64 genera. It included 46 endemic tree taxa (30 species and 16 subspecies), mainly distributed within a single biogeographical unit. -

From Genes to Genomes: Botanic Gardens Embracing New Tools for Conservation and Research Volume 18 • Number 1
Journal of Botanic Gardens Conservation International Volume 18 • Number 1 • February 2021 From genes to genomes: botanic gardens embracing new tools for conservation and research Volume 18 • Number 1 IN THIS ISSUE... EDITORS Suzanne Sharrock EDITORIAL: Director of Global Programmes FROM GENES TO GENOMES: BOTANIC GARDENS EMBRACING NEW TOOLS FOR CONSERVATION AND RESEARCH .... 03 Morgan Gostel Research Botanist, FEATURES Fort Worth Botanic Garden Botanical Research Institute of Texas and Director, GGI-Gardens NEWS FROM BGCI .... 06 Jean Linksy FEATURED GARDEN: THE NORTHWESTERN UNIVERSITY Magnolia Consortium Coordinator, ECOLOGICAL PARK & BOTANIC GARDENS .... 09 Atlanta Botanical Garden PLANT HUNTING TALES: GARDENS AND THEIR LESSONS: THE JOURNAL OF A BOTANY STUDENT Farahnoz Khojayori .... 13 Cover Photo: Young and aspiring scientists assist career scientists in sampling plants at the U.S. Botanic Garden for TALKING PLANTS: JONATHAN CODDINGTON, the Global Genome Initiative (U.S. Botanic Garden). DIRECTOR OF THE GLOBAL GENOME INITIATIVE .... 16 Design: Seascape www.seascapedesign.co.uk BGjournal is published by Botanic Gardens Conservation International (BGCI). It is published twice a year. Membership is open to all interested individuals, institutions and organisations that support the aims of BGCI. Further details available from: ARTICLES • Botanic Gardens Conservation International, Descanso House, 199 Kew Road, Richmond, Surrey TW9 3BW UK. Tel: +44 (0)20 8332 5953, Fax: +44 (0)20 8332 5956, E-mail: [email protected], www.bgci.org BANKING BOTANICAL BIODIVERSITY WITH THE GLOBAL GENOME • BGCI (US) Inc, The Huntington Library, BIODIVERSITY NETWORK (GGBN) Art Collections and Botanical Gardens, Ole Seberg, Gabi Dröge, Jonathan Coddington and Katharine Barker .... 19 1151 Oxford Rd, San Marino, CA 91108, USA. -

Chec List What Survived from the PLANAFLORO Project
Check List 10(1): 33–45, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution What survived from the PLANAFLORO Project: PECIES S Angiosperms of Rondônia State, Brazil OF 1* 2 ISTS L Samuel1 UniCarleialversity of Konstanz, and Narcísio Department C.of Biology, Bigio M842, PLZ 78457, Konstanz, Germany. [email protected] 2 Universidade Federal de Rondônia, Campus José Ribeiro Filho, BR 364, Km 9.5, CEP 76801-059. Porto Velho, RO, Brasil. * Corresponding author. E-mail: Abstract: The Rondônia Natural Resources Management Project (PLANAFLORO) was a strategic program developed in partnership between the Brazilian Government and The World Bank in 1992, with the purpose of stimulating the sustainable development and protection of the Amazon in the state of Rondônia. More than a decade after the PLANAFORO program concluded, the aim of the present work is to recover and share the information from the long-abandoned plant collections made during the project’s ecological-economic zoning phase. Most of the material analyzed was sterile, but the fertile voucher specimens recovered are listed here. The material examined represents 378 species in 234 genera and 76 families of angiosperms. Some 8 genera, 68 species, 3 subspecies and 1 variety are new records for Rondônia State. It is our intention that this information will stimulate future studies and contribute to a better understanding and more effective conservation of the plant diversity in the southwestern Amazon of Brazil. Introduction The PLANAFLORO Project funded botanical expeditions In early 1990, Brazilian Amazon was facing remarkably in different areas of the state to inventory arboreal plants high rates of forest conversion (Laurance et al. -

Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 7-30-2008 Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St. Louis Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Biology Commons Recommended Citation Sweeney, Patrick Wayne, "Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)" (2008). Dissertations. 539. https://irl.umsl.edu/dissertation/539 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. SYSTEMATICS AND FLORAL EVOLUTION IN THE PLANT GENUS GARCINIA (CLUSIACEAE) by PATRICK WAYNE SWEENEY M.S. Botany, University of Georgia, 1999 B.S. Biology, Georgia Southern University, 1994 A DISSERTATION Submitted to the Graduate School of the UNIVERSITY OF MISSOURI- ST. LOUIS In partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in BIOLOGY with an emphasis in Plant Systematics November, 2007 Advisory Committee Elizabeth A. Kellogg, Ph.D. Peter F. Stevens, Ph.D. P. Mick Richardson, Ph.D. Barbara A. Schaal, Ph.D. © Copyright 2007 by Patrick Wayne Sweeney All Rights Reserved Sweeney, Patrick, 2007, UMSL, p. 2 Dissertation Abstract The pantropical genus Garcinia (Clusiaceae), a group comprised of more than 250 species of dioecious trees and shrubs, is a common component of lowland tropical forests and is best known by the highly prized fruit of mangosteen (G. mangostana L.). The genus exhibits as extreme a diversity of floral form as is found anywhere in angiosperms and there are many unresolved taxonomic issues surrounding the genus. -
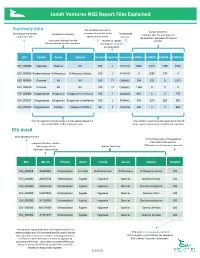
NGS Report Explained
Jonah Ventures NGS Report Files Explained Summary data The % of base pairs in the Sample identifiers Exact Sequence Variant sequence that match to the The detected Exact Sequence Variant Consensus Taxonomy The detected Numbers after the decimal point unique identifier species in the library. sequence. represent lab replicates of the same Taxonomic ranking from the Number of species sample. library matched to each sequence. matching the sequence at a given level. ESV Family Genus Species %match # Species Sequence S10001.1 S10002.1 S10003.1 S10003.2 ESV_000080 Fagaceae Quercus NA 100 7 AAAAAG… 3066 1340 5780 3462 ESV_000818 Orobanchaceae Orthocarpus Orthocarpus luteus 100 1 AAAAAG… 0 2582 249 0 ESV_000006 Poaceae NA NA 100 77 GAAAAG… 584 101 0 1039 ESV_000050 Poaceae NA NA 100 12 GAAAAG… 1328 0 0 0 ESV_000308 Polygonaceae Polygonum Polygonum humifusum 100 2 AAAAAG… 902 0 0 278 ESV_000027 Polygonaceae Eriogonum Eriogonum umbellatum 100 1 ATAAAG… 341 124 282 381 ESV_000520 Polygonaceae Fallopia Fallopia multiflora 99 1 AAAAAG… 126 0 0 994 “NA” at a taxonomic ranks means multiple species present in The number in each cell is the absolute number of the sample differ in that taxonomic rank times a given sequence was read by the sequencer. ESV detail Exact Sequence Variant The % of base pairs in the sequence that match to the species. Index identification number. that match to the species. Each sample has an Species Taxonomy 100% indicates all base pairs matched individual index number. ESV GB_ID Phylum Order Family Genus Species %match ESV_000818 -

Response of Downy Oak (Quercus Pubescens Willd.) to Climate Change: Transcriptome Assembly, Differential Gene Analysis and Targe
plants Article Response of Downy Oak (Quercus pubescens Willd.) to Climate Change: Transcriptome Assembly, Differential Gene Analysis and Targeted Metabolomics Jean-Philippe Mevy 1,*, Beatrice Loriod 2, Xi Liu 3, Erwan Corre 3, Magali Torres 2, Michael Büttner 4, Anne Haguenauer 1, Ilja Marco Reiter 5, Catherine Fernandez 1 and Thierry Gauquelin 1 1 CNRS, Aix-Marseille University, Avignon University, IRD, IMBE, 13331 Marseille, France; [email protected] (A.H.); [email protected] (C.F.); [email protected] (T.G.) 2 TGML-TAGC—Inserm UMR1090 Aix-Marseille Université 163 avenue de Luminy, 13288 Marseille, France; [email protected] (B.L.); [email protected] (M.T.) 3 CNRS, Sorbonne Université, FR2424, ABiMS platform, Station Biologique, 29680 Roscoff, France; xi.liu@sb-roscoff.fr (X.L.); erwan.corre@sb-roscoff.fr (E.C.) 4 Metabolomics Core Technology Platform Ruprecht-Karls-University Heidelberg Centre for Organismal Studies (COS) Im Neuenheimer Feld 360, 69120 Heidelberg, Germany; [email protected] 5 Research Federation ECCOREV FR3098, CNRS, 13545 Aix-en-Provence, France; [email protected] * Correspondence: [email protected]; Tel.: +33-0413550766 Received: 20 July 2020; Accepted: 1 September 2020; Published: 4 September 2020 Abstract: Global change scenarios in the Mediterranean basin predict a precipitation reduction within the coming hundred years. Therefore, increased drought will affect forests both in terms of adaptive ecology and ecosystemic services. However, how vegetation might adapt to drought is poorly understood. In this report, four years of climate change was simulated by excluding 35% of precipitation above a downy oak forest. -

Platonia Insignis Instituto Interamericano De Cooperación Para La Agricultura (IICA), 2017
BACURI Platonia insignis Instituto Interamericano de Cooperación para la Agricultura (IICA), 2017 Este documento se encuentra bajo una Licencia Creative Commons Atribución- NoComercial-CompartirIgual 3.0 Unported. Basada en una obra en www.iica.int. El Instituto promueve el uso justo de este documento. Se solicita que sea citado apropiadamente cuando corresponda. Esta publicación también está disponible en formato electrónico (PDF) en el sitio Web institucional en http://www.iica.int. Coordinación editorial: Rosanna Leggiadro Corrección de estilo: Malvina Galván Diseño de portada: Esteban Grille Diseño editorial: Esteban Grille Editores técnicos: Marília Lobo Burle Fábio Gelape Faleiro Platonia insignis Bacuri José Edmar Urano de Carvalho Walnice Maria Oliveira do Nascimento1 1. ANTECEDENTES HISTÓRICOS E CULTURAIS O bacurizeiro (Platonia insignis Mart.) é uma espécie arbórea de dupla aptidão (madeira e fruto), nativa da Amazônia Brasileira. Os frutos dessa Clusiaceae, pelo sabor e aroma agradáveis, são bastante apreciados pela população da Amazônia e de parte do Nordeste do Brasil, em particular dos Estados do Maranhão e do Piauí, onde a espécie também é encontrada espontaneamente. Em termos de sabor, o bacuri é considerado por muitos como a melhor fruta da Amazônia. Não obstante a grande demanda por essa fruta e a excelente cotação que atinge na Amazônia e nas demais áreas de ocorrência, o cultivo do bacuri- zeiro ainda é inexpressivo. Tal fato é devido às dificuldades de propagação e a longa fase jovem da planta. No início da década de 1940, Pesce (2009) já enfatizava que a planta não era cultivada, devido ao seu crescimento lento, conquanto, naquela época, os frutos eram muito procurados pelas peque- nas fábricas de doces, bastante comuns no Estado do Pará nessa época, e consumidos pela população local, ao natural e na forma de refresco, licor, doce em pasta e compota. -

Instituto Nacional De Pesquisas Da Amazônia – Inpa
INSTITUTO NACIONAL DE PESQUISAS DA AMAZÔNIA – INPA PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA PRODUÇÃO DE ISOPRENÓIDES E ESTRATÉGIAS DE ALOCAÇÃO DE RECURSOS EM ESPÉCIES ARBÓREAS DA AMAZÔNIA CENTRAL MICHELLE ROBIN CARNEIRO DE REZENDE Manaus, Amazonas Setembro/2020 1 MICHELLE ROBIN CARNEIRO DE REZENDE PRODUÇÃO DE ISOPRENÓIDES E ESTRATÉGIAS DE ALOCAÇÃO DE RECURSOS EM ESPÉCIES ARBÓREAS DA AMAZÔNIA CENTRAL Orientadora: Juliana Schietti de Almeida Co-orientadora: Eliane Gomes Alves Dissertação apresentada ao Instituto Nacional de Pesquisas da Amazônia como parte dos requisitos para obtenção do título de Mestre em Biologia (Ecologia). Manaus, Amazonas Setembro/2020 2 BANCA EXAMINADORA DA DEFESA ORAL PÚBLICA 3 FICHA CATALOGRÁFICA SINOPSE Estudou-se a correlação entre os espectros da economia de carbono - gerados a partir de traços funcionais medidos no nível da folha, da madeira, e da planta-inteira - com a emissão e o estoque de compostos secundários da classe dos isoprenóides, em 27 espécies de árvores da Amazônia Central. Palavras-chave: compostos secundários, traços funcionais, estratégias de plantas, isopreno, COVBs, floresta Amazônica 4 AGRADECIMENTOS A presente dissertação não teria sido possível sem o auxílio e a contribuição de muitas pessoas, às quais sou imensamente grata. Aos meus pais, por todo o carinho e amor, por sempre me apoiarem e me incentivarem na busca de novos sonhos e desafios. Às minhas orientadoras, Juliana Schietti e Eliane Alves, pela orientação e todo o apoio, incentivo, aprendizado e crescimento, tanto profissional quanto pessoal, ao longo desse período de mestrado; pela oportunidade de descobrir, e de me encantar, com a Ecologia Funcional e os Compostos Orgânicos Voláteis Biogênicos, e também de conhecer e trabalhar em lugares incríveis, como o sítio da torre ATTO e o Instituto Max Planck de Biogeoquímica. -

The Hyperdominant Tropical Tree Eschweilera Coriacea (Lecythidaceae) Shows Higher Genetic Heterogeneity Than Sympatric Eschweilera Species in French Guiana
Plant Ecology and Evolution 153 (1): 67–81, 2020 https://doi.org/10.5091/plecevo.2020.1565 REGULAR PAPER The hyperdominant tropical tree Eschweilera coriacea (Lecythidaceae) shows higher genetic heterogeneity than sympatric Eschweilera species in French Guiana Myriam Heuertz1,*, Henri Caron1,2, Caroline Scotti-Saintagne3, Pascal Pétronelli2, Julien Engel4,5, Niklas Tysklind2, Sana Miloudi1, Fernanda A. Gaiotto6, Jérôme Chave7, Jean-François Molino5, Daniel Sabatier5, João Loureiro8 & Katharina B. Budde1,9 1Univ. Bordeaux, INRAE, Biogeco, FR-33610 Cestas, France 2INRAE, Cirad, Ecofog, GF-97310 Kourou, French Guiana 3INRAE, URFM, FR-84914 Avignon, France 4International Center for Tropical Botany, Department of Biological Sciences, Florida International University, Miami, FL-33199, USA 5Université de Montpellier, IRD, Cirad, CNRS, INRAE, AMAP, FR-34398 Montpellier, France 6Universidade Estadual de Santa Cruz, Centro de Biotecnologia e Genética, Ilhéus, BR-45662-901, Bahia, Brazil 7Université Paul Sabatier Toulouse, CNRS, EBD, FR-31062, Toulouse, France 8University of Coimbra, Centre for Functional Ecology, Department of Life Sciences, PT-3000-456 Coimbra, Portugal 9Present address: University of Copenhagen, Forest, Nature and Biomass, Rolighedsvej 23, DK-1958 Frederiksberg C, Denmark *Corresponding author: [email protected] Background and aims – The evolutionary history of Amazonia’s hyperabundant tropical tree species, also known as “hyperdominant” species, remains poorly investigated. We assessed whether the hyperdominant Eschweilera coriacea (DC.) S.A.Mori (Lecythidaceae) represents a single genetically cohesive species, and how its genetic constitution relates to other species from the same clade with which it occurs sympatrically in French Guiana. Methods – We sampled 152 individuals in nine forest sites in French Guiana, representing 11 species of the genus Eschweilera all belonging to the Parvifolia clade, with emphasis on E. -

Systematics and Biogeography of the Clusioid Clade (Malpighiales) Brad R
Eastern Kentucky University Encompass Biological Sciences Faculty and Staff Research Biological Sciences January 2011 Systematics and Biogeography of the Clusioid Clade (Malpighiales) Brad R. Ruhfel Eastern Kentucky University, [email protected] Follow this and additional works at: http://encompass.eku.edu/bio_fsresearch Part of the Plant Biology Commons Recommended Citation Ruhfel, Brad R., "Systematics and Biogeography of the Clusioid Clade (Malpighiales)" (2011). Biological Sciences Faculty and Staff Research. Paper 3. http://encompass.eku.edu/bio_fsresearch/3 This is brought to you for free and open access by the Biological Sciences at Encompass. It has been accepted for inclusion in Biological Sciences Faculty and Staff Research by an authorized administrator of Encompass. For more information, please contact [email protected]. HARVARD UNIVERSITY Graduate School of Arts and Sciences DISSERTATION ACCEPTANCE CERTIFICATE The undersigned, appointed by the Department of Organismic and Evolutionary Biology have examined a dissertation entitled Systematics and biogeography of the clusioid clade (Malpighiales) presented by Brad R. Ruhfel candidate for the degree of Doctor of Philosophy and hereby certify that it is worthy of acceptance. Signature Typed name: Prof. Charles C. Davis Signature ( ^^^M^ *-^£<& Typed name: Profy^ndrew I^4*ooll Signature / / l^'^ i •*" Typed name: Signature Typed name Signature ^ft/V ^VC^L • Typed name: Prof. Peter Sfe^cnS* Date: 29 April 2011 Systematics and biogeography of the clusioid clade (Malpighiales) A dissertation presented by Brad R. Ruhfel to The Department of Organismic and Evolutionary Biology in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Biology Harvard University Cambridge, Massachusetts May 2011 UMI Number: 3462126 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted.