Assessment of Fluoride Contamination in Groundwater from Basara
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Courses Towards Trade in Early Andhra (With Reference to the Krishna and Godavari Valleys) Dr
American International Journal of Available online at http://www.iasir.net Research in Humanities, Arts and Social Sciences ISSN (Print): 2328-3734, ISSN (Online): 2328-3696, ISSN (CD-ROM): 2328-3688 AIJRHASS is a refereed, indexed, peer-reviewed, multidisciplinary and open access journal published by International Association of Scientific Innovation and Research (IASIR), USA (An Association Unifying the Sciences, Engineering, and Applied Research) Courses towards Trade in Early Andhra (With reference to the Krishna and Godavari valleys) Dr. G. Mannepalli Faculty Member, Dept. of History & Archaeology, Acharya Nagarjuna University, Guntur, Andhra Pradesh, INDIA. I. Introduction: As for as the economic potentiality of the Krishna Valley consisting of a large number of sites presenting a well progress agriculture serves as a clear background of the corresponding progress of arts and crafts, leading to overseas trade and commerce. It is somewhat clear that the archaeology of the Andhra Pradesh and the rest of the South India falls roughly into two main stages. The first one which closed about 1000 B.C. was Neolithic- Chalcolithic which witnessed the building up of an effective rural-agricultural base. During the second phase, beginning from about 1000 B.C. this rural agricultural base was strengthened and further activised by the use of iron, without however suffering any break in continuity in so far as rural agriculture was concerned. But the very extensive use of iron may have brought about certain socio-economic changes as well which ante-dated the growth of urban centres. We do not have much evidence in this regard, but the transformation from hoe to regular plough cultivation in the fertile valleys of the Godavari and the Krishna may be regarded as one of the main important items in this change. -

Nalgonda District, Andhra Pradesh
For Official Use Only CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES GOVERNMENT OF INDIA GROUND WATER BROCHURE NALGONDA DISTRICT, ANDHRA PRADESH SOUTHERN REGION HYDERABAD September 2013 CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES GOVERNMENT OF INDIA GROUND WATER BROCHURE NALGONDA DISTRICT, ANDHRA PRADESH (AAP-2012-13) BY D. MOHANTA ASST. HYDROGEOLOGIST SOUTHERN REGION BHUJAL BHAWAN, GSI Post, Bandlaguda NH.IV, FARIDABAD-121001 Hyderabad-500068 HARYANA, INDIA Andhra Pradesh Tel: 0129-2418518 Tel: 040-24225201 Gram: Bhumijal Gram: Antarjal NALGONDA DISTRICT AT A GLANCE Sl. GENERAL INFORMATION No 1 Geographical Area (2011 census) 14200 sq.km Headquarters Nalgonda Location North latitudes 16° 25’ and 17° 50’ East longitudes 78° 40’ and 80° 05’ Administrative Divisions Mandals-59, Revenue Divisions-4 at Bhongir, (As on 31/03/2010) Nalgonda, Miryalguda and Suryapet No. of Revenue Villages 1161 Population (2011 census) 3483648 Population Density 245/ sqkm 2 Land Use (2012) Forest 83073 Ha Barren and Uncultivable 121531 Ha Cultivable waste 29146 Ha Net Area Sown 573291 Ha 3 Irrigation (2012) Major Projects Nagarjun Sagar Medium Projects 1. Musi, 2. AMRP (A. Madhav Reddy Project) 3. Dindi Gross Irrigated Area 408093 Ha Net Irrigated Area 297796 Ha 4 RAINFALL Normal Annual Rainfall (Mandalwise) Minimum 540.00 mm (M- Pedda Adiserlapalli) to Maximum 932.00mm (M-Thirumalgiri) Annual rainfall (2012) 674 mm 5 Geomorphology Major Drainage Two; Musi and Dindi 6 Soil Type 1. Red soils, 2. Black soils 3. Alkaline soils and 4. Alluvium -
Inventory of Soil & Land Resources Mapping of Khammam District of Telangana State Using Remote Sensing Techniques
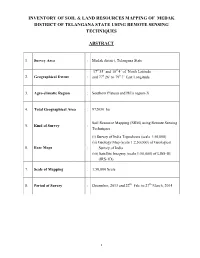
INVENTORY OF SOIL & LAND RESOURCES MAPPING OF MEDAK DISTRICT OF TELANGANA STATE USING REMOTE SENSING TECHNIQUES ABSTRACT 1. Survey Area : Medak district, Telangana State 170 35’ and 180 4’ of North Latitude 2. Geographical Extent : and 770 26’ to 790 7’ East Longitude 3. Agro-climatic Region : Southern Plateau and Hills region-X 4. Total Geographical Area : 972030 ha Soil Resource Mapping (SRM) using Remote Sensing 5. Kind of Survey : Techniques (i) Survey of India Toposheets (scale 1:50,000) (ii) Geology Map (scale 1:2,50,000) of Geological 6. Base Maps : Survey of India (iii) Satellite Imagery (scale 1:50,000) of LISS-III (IRS-1D) 7. Scale of Mapping : 1:50,000 Scale 8. Period of Survey : December, 2013 and 22th Feb. to 27th March, 2014 i 9. Mapping unit wise soil association and their extent. S No. Mapping Units Soil Association AREA(Ha.) 1 ALb1a1 Arepalli - Jublee 27654 2 BAr5d1 Nagwar - Kankol 627 3 BAu4d1 Kankol –Nagwar -Singtam 8057 4 BAv2a1 Lingampalli - Halagiri 12207 5 BAv2a2 Kaveli - Halagiri 54246 6 BAv2d1 Kankol - Singtam 18102 7 BAv3d1 Singtam - Kankol 21131 8 BAw2a1 Khusnur 37289 9 DLu4d1 Pegudapalli 1209 10 GRn6c1 Bhimaram 10526 11 GRu4c1 Kurmapalli - Jillela 21070 12 GRu4d1 Jillela - Kurmapalli 6106 13 GRv2a1 Gundi - Gollapalli 109204 14 GRv2a2 Paidipalli - Saidapur 61818 15 GRv2a3 Pathipaka - Pragnapur 12533 16 GRv2a4 Thimmapur – Gollapalli - Gundi 85635 17 GRv2a5 Suraram - Thimmapur 54967 18 GRv2b1 Birur 14597 19 GRv2d1 Gajwel - Kistapur 72375 20 GRv2d2 Kistapur - Kondapalli 4810 21 GRv3a1 Thimmapur - Bollaram 9146 22 GRv3c1 Thotapalli - Kurmapalli 47719 23 GRv3d1 Maddimilla - Thimmapur 50925 24 GRw1a1 Isojipeta – Rajakkapalli - Manakondur 100138 25 LAr5d1 Guntepalli 15 26 LAu4d1 Ganeshpur 3357 27 LAv2a1 Govindpur - Kohir 11251 28 LAv2a2 Kohir - Mannapur 6435 29 LAv2d1 Digwal - Nallapalli 13727 30 LAv3c1 Shekapur - Chintalghat 2850 31 LAv3d1 Nallapalli - Digwal 16457 32 Reservoir 13436 33 River 9229 34 Tank 33566 35 ROC 1474 36 Hab. -

Use of Various Bio-Fencing Plants in the Control of Human Diseases by the Lambada Tribe Inhabiting Nalgonda District, Andhra Pradesh, India
Ethnobotanical Leaflets 12: 520-23. 2008. Use of Various Bio-Fencing Plants in the Control of Human Diseases by the Lambada Tribe Inhabiting Nalgonda District, Andhra Pradesh, India A. Vijaya Bhasker Reddy Department of Botany P.G.College of Science Saifabad, Hyderabad –500004, India Email: [email protected] Issued 25 July 2008 Abstract The present paper deals with 16 bio-fencing plants, which are being used for control of various diseases in human beings by Lambada tribes of Nalgonda District, Andhra Pradesh. The biomedicines are collected from the plants, which are used as fencing plants to their agricultural fields. This work is being carried out in collaboration with local Lambada tribes of Manchya Naik Thanda of Nalgonda district. The documented ethno medicine information was indexed by plant name, family, local name and uses. Key Words: Lambada Tribe, Fencing plants, Nalgonda District, Andhra Pradesh. Introduction Nalgonda district lies between 170 50’ N latitude and 780 10’ and 800 5’ S longitude. The district is bounded on the north by Medak and Warangal districts, on the South by Mahabubnagar and Guntur districts, on the West by the Hyderabad and Ranga Reddy districts and on the East by Khammam and Krishna districts. The district has geographical area of 14,240 sq. km. With a total population of 30,80,000. The tribal population in the district is 10%. The forest occupies 83,000 hectares area; these are mostly of tropical thorny type. Two important tribal settlements are seen in this district. They are Lambada and Erukala. This work is concentrated on Lambada tribe living at Manchya Naik Thanda, Mattampally Mandal of Nalgonda District. -

GOVERNMENT of TELANGANA ABSTRACT Public Services
GOVERNMENT OF TELANGANA ABSTRACT Public Services – Formation /Reorganization of New Districts, Revenue Divisions and Mandals in Telangana State – Re-organization of Circles/Divisions/Sub- Divisions/Mandals in all cadres - Orders – Issued. PANCHAYAT RAJ & RURAL DEVELOPMENT (PR.I) DEPARTENT G.O.Ms.No.71 Dt:11.10.2016 Read the following:- 1. G.O.Ms.No.5, PR&RD(Estt.I) Dept. Dt:16.01.2015 and subsequent amendments, G.O.Ms.No.45, dt:23.5.2015, G.O.Ms.No.59, dt:31.7.2015 and G.O.Ms.No.6, dt:13.01.2016. 2. G.O.Ms.No.221 to 250, Revenue (DA-CMRF) Department, dt:11.10.2016 3. G.O.Ms.No.144, Finance (HRM.I) Department, dt:11.10.2016 4. From the E-in-C, PR, Hyderbad Letter No.B-II/Reorg.district/ 338/2016, Dt.17.9.2016, Dt:29.9.2016 & Dt:08.10.2016. ORDER: In the reference first read above Government have issued orders rationalising the PRI, PIU & Q C wings for effective implementation of works programme in PRED to achieve the targets of the Govt. 2. In the reference second read above Government of Telangana have issued notifications for formation/reorganization of Districts, Divisions and Mandals in the State of Telangana for better administration and development of areas concerned. 3. In the reference 3rd read above, Government have issued orders re- distributing cadre strength among (30) districts. 4. In the reference fourth read above the Engineer-in-Chief, PR has submitted proposals for re-organization of PRED to be co-terminus with the new districts jurisdiction and to change the nomenclature of Superintending Engineer, PR as Regional officer and Executive Engineer of the District Office as District Panchayat Raj Engineer (DPRE). -

Committee for Consultations on the Situation in Andhra Pradesh
COMMITTEE FOR CONSULTATIONS ON THE SITUATION IN ANDHRA PRADESH REPORT December 2010 THE COMMITTEE CHAIRPERSON Shri Justice B N Srikrishna (Retd.) Former Judge, Supreme Court of India MEMBER SECRETARY Shri Vinod Kumar Duggal, IAS (Retd.) Former Home Secretary, Government of India MEMBERS Prof (Dr.) Ranbir Singh Vice Chancellor, National Law University, Delhi Dr. Abusaleh Shariff Chief Economist /Senior Fellow, National Council of Applied Economic Research, Delhi Prof (Dr.) Ravinder Kaur Department of Humanities and Social Sciences, IIT, Delhi The Inter State Council Secretariat (ISCS) provided full secretarial assistance including technical and budgetary support to the Committee C O N T E N T S VOLUME - I Prologue i Approach and Methodology iv Acknowledgements xii List of Tables, Figures, Appendices xvii Abbreviations xxix Chapter 1 Developments in Andhra Pradesh-A Historical Background 1 Chapter 2 Regional Economic and Equity Analysis 63 Chapter 3 Education and Health 125 Chapter 4 Water Resources, Irrigation and Power Development 177 Chapter 5 Public Employment Issues 245 Chapter 6 Issues Relating to Hyderabad Metropolis 295 Chapter 7 Sociological and Cultural Issues 341 Chapter 8 Law & Order and Internal Security Dimensions 423 Chapter 9 The Way Forward 425 VOLUME - II Appendices 1-173 Index 174 “In ages long past a great son of India, the Buddha, said that the only real victory was one in which all were equally victorious and there was defeat for no one. In the world today that is the only practical victory; any other way will lead to disaster”. Pt. Jawaharlal Nehru speaking on „Disputes and Discord‟ in the United Nations General Assembly on October 3, 1960 Prologue It has not been an easy task. -

Annual Report 2011-12 Summary
Dr.YSRHU, Annual Report, 2011-12 Published by Dr.YSR Horticultural University Administrative Office, P.O. Box No. 7, Venkataramannagudem-534 101, W.G. Dist., A.P. Phones : 08818-284312, Fax : 08818-284223 E-mail : [email protected], [email protected] URL : www.drysrhu.edu.in Compiled and Edited by Dr. B. Srinivasulu, Registrar & Director of Research (FAC), Dr.YSRHU Dr. M.B.Nageswararao, Director of Extension, Dr.YSRHU Dr. M.Lakshminarayana Reddy, Dean of Horticulture, Dr.YSRHU Dr. D.Srihari, Dean of Student Affairs & Dean PG Studies, Dr.YSRHU Lt.Col. P.R.P. Raju, Estate Officer, Dr.YSRHU Dr.B.Prasanna Kumar, Deputy COE, Dr.YSRHU All rights are reserved. No part of this book shall be reproduced or transmitted in any form by print, microfilm or any other means without written permission of the Vice-Chancellor, Dr.Y.S.R. Horticultural University, Venkataramannagudem. Printed at Dr.C.V.S.K.SARMA, I.A.S. VICE-CHANCELLOR Dr.Y.S.R. Horticultural University & Agricultural Production Commissioner & Principal Secretary to Government, A.P. I am happy to present the Fourth Annual Report of Dr.Y.S.R. Horticultural University (Dr.YSRHU). It is a compiled document of the university activities during the year 2011-12. Dr.YSR Horticultural University was established at Venkataramannagudem, West Godavari District, Andhra Pradesh on 26th June, 2007. Dr.YSR Horticultural University second of its kind in the country, with the mandate for Education, Research and Extension related to horticulture and allied subjects. The university at present has 4 Horticultural Colleges, 5 Polytechnics, 25 Research Stations and 3 KVKs located in 9 agro-climatic zones of the state. -

Telangana State Information Commission
Telangana State Information Commission (Under Right to Information Act, 2005) D.No.5-4-399, Samachara Hakku Bhavan (Old ACB Building), Mojam-jahi-Market, Hyderabad – 500 001 Phone: 24740666 Fax: 24740592 Complaint No: 4899/SIC-BM/2019 Date: 10-12-2019 Complainant : Sri. B. Ravi, Hanamkonda, Warangal Urban District. Respondent : Public Information Officer (U/RTI Act, 2005) O/o the District Fisheries Officer, Collectorate Office, Nalgonda District. Order Sri. B. Ravi, Hanamkonda, Warangal Urban District has filed a complaint dated 24-04-2019 which was received by this Commission on 26-04-2019 for not complying the Commission order in appeal No: 9483/SIC-BM/2018 dated: 28-02-2019 and for not getting the information sought by him through 6(1) application dated: 16-04-2018 from the PIO / O/o the District Fisheries Officer, Collectorate Office, Nalgonda District. The complaint was taken on file and notices were issued to both the parties for hearing on 10-12-2019. The case is called on 10-12-2019. The complainant is present and stated that the PIO has not furnished the information. The PIO / Smt. R. Nirmala, Superintendent, O/o the District Fisheries Officer, Nalgonda District is present and submitted that the information sought by the complainant was furnished vide letter No: RTI/A/2018 dated: 06-03-2019 and filed copies of the information furnished before the Commission. Heard both the parties and perused the material papers available on record. The Commission observed that the PIO has failed to submit the postal evidence of having furnishedTSIC the information by post. -

District Wise News Papers Empanelled List
DISTRICT-WISE NEWSPAPERS LIST EMPANELLED IN I&PR DEPT. AS ON 16.1.2010 Srikakulam District S.No. Name of the paper Edition 1 Kalingaseema Srikakulam 2People's Vision Srikakulam 3 Satyam Srikakulam 4 Visesha Varthala Janasri Surya Srikakulam 5 Vijayabhanu Srikakulam 6 Neti Andhra Srikakulam Vizianagaram District S.No. Name of the paper Edition 1 Grameena Vedika Vizianagaram 2 Vizianagaram Times Vizianagaram Visakhapatnam District S.No. Name of the paper Edition 1 Visakhasamacharam Vizag 2 Leader Vizag 3 Surya Prabha Vizag 4 Vijayabhanu Vizag 5 Vizag Reporter Vizag 6 Andhra Voice Vizag 7 Neti Andhra Vizag 8 People's Vision Vizag 9 Pledge Vizag 10 Bay News Vizag 11 Greater News Vizag 12Vision of the People Vizag 13 Teluguvaaram Vizag 14 Metro Evenings Vizag 15Gopi Krishna Vizag 16 Krishna Patrika Vizag East Godavari District S.No. Name of the paper Edition 1 Visakhasamacharam Rajahmundry 2 Leader Rajahmundry 3 Janaspandana Kakinada 4 Godavari Amalapuram 5 Vennela Rajahmundry 6 Aruna Kakinada 7 Circar Express Kakinada 8People's Vision Rajahmundry 9 Jana Jeevana Vikasa Velugu Kakinada 10 Vijayabhanu Kakinada 11Kostavani Rajahmundry West Godavari District S.No. Name of the paper Edition 1Gopi Krishna Eluru 2Eluru Times Eluru 3State Times Eluru 4 Helapuri News Eluru 5 Ratnagarbha Eluru 6Vasista Times Eluru 7Aavinithiki Sankelu Eluru 8 Nethajee Eluru 9 Jayakethanam Eluru 10 Jeevana Rekha Eluru 11 Visakhasamacharam Eluru 12 Andhra Dairy Eluru 13 Vennela Tadepalligudem Krishna District S.No. Name of the paper Edition 1 Janatha Vijayawada 2 Swarnandhra Vijayawada 3 Pledge Vijayawada 4 Today Freedom Vijayawada 5 Skyline Vijayawada 6 News Boom Vijayawada 7 Krishna Patrika Vijayawada 8 Neti Manadesam Vijayawada 9 Udayabharatham Vijayawada 10 Rakshana Vijayawada 11 Vijayandhra Bilingula Vijayawada 12 Citizen's Evening Vijayawada 13 Andhra Voice Vijayawada 14 Vijayabhanu Vijayawada 15 Sena Kaikaluru Guntur District S.No. -

In the Court of the Sessions Judge
1 IN THE COURT OF I ADDITIONAL DISTRICT AND SESSIONS JUDGE AT KARIMNAGAR. Present :- DR. S. SRINIVAS REDDY, I ADDL. SESSIONS JUDGE, KARIMNAGAR Friday, this the 11th day of December, 2020 CRL. MISC. PETITION NO. 741 OF 2020 IN CR. NO. 190 OF 2020 OF P.S., ELLANTHAKUNTA Between:- Edire Ravi, S/o Pochaiah, aged 23 Years, Caste: Madiga, R/o Ananthagiri village of Ellanthakunta Mandal of Rajanna Sircilla district. …Petitioner/Accused And The State through SHO: P.S. Ellanthakunta represented by Additional Public Prosecutor, Sessions Court, Karimnagar. …Respondent/Complainant. PETITION FILED UNDER SECTION 439 of Cr.P.C Offences punishable under Sections 366-A, 417, 420, 493, 496, 376 (2) (n) of Indian Penal Code and Section 6 of Protection of Children from Sexual Offences Act, 2012 (for Short “POCSO Act”) read with Section 5 (I) of POCSO Act, 2012. This petition is coming before me today i.e., on 11-12-2020 for hearing by Sri J. Satyanarayana Rao, Advocate for the petitioner/accused and the learned Additional Public Prosecutor for State and having heard them, this Court made the following :- ORDER 1. This is the second bail petition filed under Section 439 of Cr.P.C by the petitioner/accused in Cr.No. 190 of 2020 of P.S. Ellanthakunta for the offences under Sections 366-A, 417, 420, 493, 496, 376 (2) (n) of Indian Penal Code and Section 6 of Protection of Children from Sexual Offences Act, 2012 (for Short “POCSO Act”) read with Section 5 (I) of POCSO Act, 2012. De facto complainant notice served, none represented. -

List of Care and Support Centres Sn State District
LIST OF CARE AND SUPPORT CENTRES SN STATE DISTRICT NAME OF THE ORGANISATION 1 ANDHRA ADILABAD ADILA ADHARSHA HIV POSITIVE PRADESH PEOPLES WELFARE SOCIETY 2 ANDHRA ANANTPUR ANANTHA NETWORK OF POSITIVES PRADESH 3 ANDHRA ANANTPUR ANANTHA NETWORK OF POSITIVES PRADESH 4 ANDHRA CHITTOOR ROPES RURAL ORGANIZATION FOR PRADESH POVERTY ERADICATION SERVICES 5 ANDHRA CHITTOOR NETWORK OF CHITTOOR POSITIVE PRADESH PEOPLE 6 ANDHRA CUDDAPAH NETWORK OF KADAPA PEOPLE PRADESH LIVING WIITH HIV/AIDS 7 ANDHRA CUDDAPAH PAVITHRA MYTHRI SANGAM PRADESH 8 ANDHRA EAST GODAVARI COASTAL NETWORK OF POSITIVE PRADESH PEOPLE 9 ANDHRA EAST GODAVARI KONASEEMA SEVA MANDALI PRADESH 10 ANDHRA EAST GODAVARI COMMUNITY HEALTH AWARENESS PRADESH AND NATURAL GREEN ENVIRONMENT SOCIETY CHANGES 11 ANDHRA GUNTUR SOCIAL EDUCATIONAL AND PRADESH ECONOMIC DEVELOPMENT SOCIETY SEEDS 12 ANDHRA GUNTUR RURAL ENVIRONMENT AND PRADESH EDUCATION DEVELOPMENT SOCEITY 13 ANDHRA GUNTUR SOCIETY FOR WELFARE OF HIV PRADESH INFECTED PEOPLE 14 ANDHRA HYDERABAD NETWORK OF HIV POSITIVE PRADESH PEOPLE NHP+ 15 ANDHRA HYDERABAD HIV OF POSITIVE PEOPLE PRADESH EFFICENCY SOCIETY 16 ANDHRA KARIMNAGAR KARIMNAGAR AASHA JYOTHI HIV PRADESH POSITIVE PEOPLES WELFARE SOCIETY 17 ANDHRA KARIMNAGAR KARIMNAGAR AASHA JYOTHI HIV PRADESH POSITIVE PEOPLES WELFARE SOCIETY 18 ANDHRA KHAMMAM ASHA POSITIVE PEOPOLE PRADESH ASSOCIATION 19 ANDHRA KHAMMAM ASHA POSITIVE ASSOCIATION PRADESH 1 LIST OF CARE AND SUPPORT CENTRES SN STATE DISTRICT NAME OF THE ORGANISATION 20 ANDHRA KRISHNA RAVICHERLA INTEGRATED PRADESH DEVELOPMENT AND EDUCATION -

Women's Farming Collectives: an Inquiry Into the Resource Sharing
Sociology and Anthropology 6(3): 283-296, 2018 http://www.hrpub.org DOI: 10.13189/sa.2018.060302 Women’s Farming Collectives: An Inquiry into the Resource Sharing Patterns across 3 Districts of Maharashtra to Provide Evidence for Sustainable i Environmental Collective Action Roshan Rathodii Society for Promoting Participatory Ecosystem Management (SOPPPECOM), India Copyright©2018 by authors, all rights reserved. Authors agree that this article remains permanently open access under the terms of the Creative Commons Attribution License 4.0 International License Abstract India is an agrarian country where, 80% of as against only 63% of men (Dand et al, 2015)[1].It is its agricultural labour is performed by women. Yet, she is significant to notice that women even though barely own a not recognized as a farmer as being a farmer is national average of only 12% of operational land holding synonymous to owning agricultural land. The land have over the years been extensively involved in different ownership statistics are obscure for women with only 12% kinds of farming operations such as the production of of the operational land holding in India and 15% in the state major grains and millers, land preparation, seed selection, of Maharashtra in particular. This study through its in depth sowing, applying manure, fertilizer and pesticide, weeding, understanding of women’s farming collectives in the 3 transplanting, threshing, winnowing and harvesting. But districts of Ratnagiri, Sindhudurg and Beed in the state of despite this increased involvement in the farming activities, Maharashtra provides insights in the functioning, resource women are as close to ignored in the decision making sharing patterns with respect to land, labour, seeds and process of farming.